No CrossRef data available.
Published online by Cambridge University Press: 25 November 2020
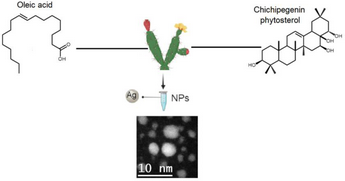
In the current work, we compared the green synthesis of silver nanoparticles (AgNP) using plant extracts, a promising methodology against the use of chemical reducers, such as oleic acid and oleylamine. The advantages of green synthesis are one-step method, economic and ecological while the advantages of classic synthesis methods are high nanoparticle performance, homogeneity in size and smaller average sizes. With this work we want to demonstrate that plant extracts with specific mixtures of chemical compounds can obtain smaller average sizes with greater homogeneity in nanoparticles compared to the use of classical synthesis. Myrtillocactus geometrizans was used as a polar plant extract, which was selected by the chemical components contained in the extract. Phytosterols, oleic acid and betalains contained in Myrtillocactus geometrizans are biomolecules responsible for the reduction and stability of AgNP below 5 nm. TEM analysis of the green synthesis of nanoparticles revealed the formation of spherical particles with an average diameter of 5 nm and with preferential crystallographic directions of the silver plane [111].