Published online by Cambridge University Press: 05 July 2018
Zigrasite, ideally MgZr(PO4)2(H2O)4, is a new secondary phosphate mineral from the giant 1972 gem tourmaline-bearing pocket at the Dunton Quarry, Newry, Oxford County, Maine, USA. It occurs as subhedral blocky grains sometimes exceeding 1 mm in maximum dimension and perched on tourmaline. These grains are complex aggregates ofthree distinct phases, zigrasite and two unnamed phases: the Ca analogue of zigrasite, CaZr(PO4)2(H2O)4, and Zr(PO3OH)2(H2O)4. Zigrasite is associated with tourmaline, microcline, quartz, albite, beryl, amblygonite-montebrasite, childreniteeosphorite and apatite, and crystallized as one of the latest minerals during pocket formation. It is offwhite to pale yellow or light tan, translucent with a white streak and a vitreous lustre, and shows light blue to pale yellow cathodoluminescence. Mohs hardness is 3 and the measured and calculated densities are 2.76(4) and 2.66 g/cm3. The mineral has imperfect cleavage in two directions, parallel to (010) and (001), shows no parting, is brittle and has a hackly fracture. In transmitted light, it is colourless and non-pleochroic, biaxial negative with α 1.597(1), β 1.622 (1), γ 1.635 (1), with 2V(meas) = 65.5(4)º and 2V(calc.) = 71º. Zigrasite is triclinic, P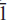 , a 5.3049(2) Å, b 9.3372(4) Å, c 9.6282(5) Å, α 97.348(1)º, β 91.534(1)º, γ 90.512(4)º, V 472.79(5) Å3, Z = 2. The seven strongest lines in the X-ray powder diffraction pattern are as follows: d (Ê), I, (hkl): 9.550, 100, (001); 4.108, 70,
, a 5.3049(2) Å, b 9.3372(4) Å, c 9.6282(5) Å, α 97.348(1)º, β 91.534(1)º, γ 90.512(4)º, V 472.79(5) Å3, Z = 2. The seven strongest lines in the X-ray powder diffraction pattern are as follows: d (Ê), I, (hkl): 9.550, 100, (001); 4.108, 70, 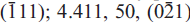 ; 4.008, 50, (111); 4.589, 40, (110); 3.177, 40, (112); 3.569, 30, (0
; 4.008, 50, (111); 4.589, 40, (110); 3.177, 40, (112); 3.569, 30, (0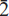 2), 2.660, 30, (200),(11),(130); 3.273, 20, (
2), 2.660, 30, (200),(11),(130); 3.273, 20, ( 12). Chemical analysis by electron microprobe gave P2O5 37.59, ZrO2 32.27, HfO2 0.34, FeO 0.20, MgO 10.37, ZnO 0.17, F 0.13, LOI 18.60, less O:F 0.05 = total 99.62 wt.%. The resulting empirical formula is (
12). Chemical analysis by electron microprobe gave P2O5 37.59, ZrO2 32.27, HfO2 0.34, FeO 0.20, MgO 10.37, ZnO 0.17, F 0.13, LOI 18.60, less O:F 0.05 = total 99.62 wt.%. The resulting empirical formula is (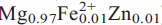 –)Σ=0.99(Zr0.99Hf0.01)Σ=1.00 P2.00O8(H2O)4 on the basis of12 O atoms with H2O = 4 per formula unit from crystal-structure analysis, and the end-member formula is MgZr(PO4)2(H2O)4.
–)Σ=0.99(Zr0.99Hf0.01)Σ=1.00 P2.00O8(H2O)4 on the basis of12 O atoms with H2O = 4 per formula unit from crystal-structure analysis, and the end-member formula is MgZr(PO4)2(H2O)4.
Please note a has been issued for this article.