Published online by Cambridge University Press: 16 November 2022
Zaykovite, ideally Rh3Se4, is a new mineral, the first natural rhodium selenide. It was discovered in the assemblages of platinum-group minerals from the Kazan gold placer, South Urals, Russia. The mineral occurs as crystals up to 40 μm in size within the grains of Pt3Fe alloy, in association with unnamed Pd–Sb–Te phase and Au–Pd alloy. In reflected light, zaykovite has a grey colour with bluish-greenish tint; it shows weak bireflectance and anisotropy. Reflectance values [Rmax/Rmin (%) for COM approved wavelengths (nm)] are: 30.1/29.3(470), 32.2/31.0(546), 33.4/32.0(589) and 35.1/33.7(650). The chemical composition corresponds to the empirical formula (Rh2.26Pt0.46Ir0.25Ru0.01Pd0.01Fe0.01)Σ3.00(Se2.77S1.21Te0.02)Σ4.00 Zaykovite is monoclinic, space group C2/m, a = 10.877(1), b = 11.192(1), c = 6.4796(6) Å, β = 108.887(2)°, V = 746.3(1) Å3, Z = 6 and Dcalc = 8.32 g cm–1. The crystal structure has been solved and refined to R1 = 0.016 based on 858 unique observed reflections. The strongest lines of the powder X-ray diffraction pattern [d(Å), (I), (hkl)] are: 5.43(37)($\bar{1}$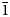 11), 3.275(75)(310), 3.199(100)($\bar{1}$
11), 3.275(75)(310), 3.199(100)($\bar{1}$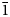 31), 3.061(87)(002), 2.568(62)(400), 2.545(41)(041), 3.413(34)($\bar{2}$
31), 3.061(87)(002), 2.568(62)(400), 2.545(41)(041), 3.413(34)($\bar{2}$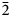 41) and 1.697(34)(441). Zaykovite is a Se analogue of kingstonite, Rh3S4. A continuous series of solid solutions between kingstonite and zaykovite was encountered in the samples from the Kazan placer. The possible sources of this unique Rh–Se mineralisation in the South Urals could be serpentinised dunite–harzburgite or gabbro–clinopyroxenite–dunite complexes in the vicinity.
41) and 1.697(34)(441). Zaykovite is a Se analogue of kingstonite, Rh3S4. A continuous series of solid solutions between kingstonite and zaykovite was encountered in the samples from the Kazan placer. The possible sources of this unique Rh–Se mineralisation in the South Urals could be serpentinised dunite–harzburgite or gabbro–clinopyroxenite–dunite complexes in the vicinity.
Associate Editor: František Laufek