Published online by Cambridge University Press: 13 December 2019
The new mineral yarzhemskiite, K[B5O7(OH)2]⋅H2O, was found in a halite–sylvite evaporite rock at the Chelkar salt dome, Western Kazakhstan Region, Kazakhstan. It is also associated with carnallite, polyhalite, gypsum, strontioginorite, satimolite and quartz. Yarzhemskiite occurs as separate thick tabular, short prismatic or equant crystals up to 0.5 mm × 0.7 mm × 1 mm and grains having irregular outlines up to 1 mm × 1.5 mm × 2 mm. The mineral is transparent, colourless, with vitreous lustre. It is brittle, the Mohs’ hardness is ca 2½. Cleavage is perfect on {100}. Dmeas is 2.13(1) and Dcalc is 2.112 g cm–3. Yarzhemskiite is optically biaxial (+), α = 1.484(2), β = 1.508(2), γ = 1.546(2), 2Vmeas = 75(10)° and 2Vcalc = 80°. Chemical composition (wt.%, electron microprobe, H2O was calculated by stoichiometry) is: Na2O 0.01, K2O 17.84, CaO 0.07, B2O3 67.21, H2Ocalc 13.91, total 99.04. The empirical formula based on 10 O atoms per formula unit is K0.98B5.005O7(OH)2⋅H2O. Yarzhemskiite is monoclinic, P21/c, a = 9.47340(18), b = 7.52030(16), c = 11.4205(2) Å, β = 97.3002(17)°, V = 807.03(3) Å3 and Z = 4. The strongest reflections of the powder XRD pattern [d,Å(I,%)(hkl)] are: 9.39(86)(100), 4.696(41)(200), 3.296(18)(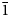 $\bar{1}$13), 3.130(19)(022, 300), 2.935(42)(220), 2.898(100)(
$\bar{1}$13), 3.130(19)(022, 300), 2.935(42)(220), 2.898(100)(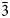 $\bar{3}$02,
$\bar{3}$02, 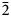 $\bar{2}$21, 310), 2.832(56)(004) and 1.867(18)(
$\bar{2}$21, 310), 2.832(56)(004) and 1.867(18)(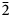 $\bar{2}$25). The crystal structure was solved based on single-crystal X-ray diffraction data, R1 = 3.36%. The structure contains infinite chains built by boron-centred polyhedra. The basic structural unit of the chain is a double ring B5O7(OH)2 consisting of one BO4 tetrahedron and four BO3 triangles. K+ cations centre ten-fold polyhedra which form, together with the borate chains [B5O7(OH)2]–∝, layers linked with each other only via H bonds. The mineral is named in honour of the Russian geologist, petrologist and mineralogist Yakov Yakovlevich Yarzhemskii (1901–?), a specialist in petrology of evaporite rocks and mineralogy and genesis of boron deposits related to evaporites.
$\bar{2}$25). The crystal structure was solved based on single-crystal X-ray diffraction data, R1 = 3.36%. The structure contains infinite chains built by boron-centred polyhedra. The basic structural unit of the chain is a double ring B5O7(OH)2 consisting of one BO4 tetrahedron and four BO3 triangles. K+ cations centre ten-fold polyhedra which form, together with the borate chains [B5O7(OH)2]–∝, layers linked with each other only via H bonds. The mineral is named in honour of the Russian geologist, petrologist and mineralogist Yakov Yakovlevich Yarzhemskii (1901–?), a specialist in petrology of evaporite rocks and mineralogy and genesis of boron deposits related to evaporites.
Associate Editor: Sergey V Krivovichev