Article contents
X-ray and electron diffraction study of penfieldite: average structure and multiple cells
Published online by Cambridge University Press: 05 July 2018
Abstract
Penfieldite is a lead hydroxychloride mineral with composition Pb2Cl3(OH). It belongs to the hexagonal system, space group P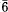 , a = 11.393(3), c = 4.024(1) Å. The 4 Å c parameter corresponds to the basic sub-cell, whereas modulated structures are known with a true c axis 12 times longer. The average crystal structure of penfieldite has been solved with direct methods and refined to Rw = 0.041 for 871 refections collected with Mo-Kα radiation. The chemical and structural relationships between penfeldite and laurelite, Pb2F3(F,Cl,OH), are briefly discussed. An electron diffraction study of penfieldite revealed the occurrence, besides the common modulated structure with C = 12c, of domains with a 15c periodicity. Moreover, a 9c periodicity has been observed in crystals heated at 180°C Penfieldite is quickly destroyed above 200°C.
, a = 11.393(3), c = 4.024(1) Å. The 4 Å c parameter corresponds to the basic sub-cell, whereas modulated structures are known with a true c axis 12 times longer. The average crystal structure of penfieldite has been solved with direct methods and refined to Rw = 0.041 for 871 refections collected with Mo-Kα radiation. The chemical and structural relationships between penfeldite and laurelite, Pb2F3(F,Cl,OH), are briefly discussed. An electron diffraction study of penfieldite revealed the occurrence, besides the common modulated structure with C = 12c, of domains with a 15c periodicity. Moreover, a 9c periodicity has been observed in crystals heated at 180°C Penfieldite is quickly destroyed above 200°C.
- Type
- Mineralogy
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1995
References
References
References
- 8
- Cited by


