Published online by Cambridge University Press: 31 March 2020
The new mineral uranoclite (IMA2020-074), (UO2)2(OH)2Cl2(H2O)4, was found in the Blue Lizard mine, San Juan County, Utah, USA, where it occurs as tightly intergrown aggregates of irregular yellow crystals in a secondary assemblage with gypsum. The streak is very pale yellow and the fluorescence is bright green–white under 405 nm ultraviolet light. Crystals are translucent with vitreous lustre. The tenacity is brittle, the Mohs hardness is ~1½, the fracture is irregular. The mineral is soluble in H2O and has a calculated density of 4.038 g⋅cm–3. Electron microprobe analyses provided (UO2)2(OH)2.19Cl1.81(H2O)4. The six strongest powder X-ray diffraction lines are [dobs Å(I)(hkl)]: 8.85(38)(002), 5.340(100)(200, 110), 5.051(63)($\bar{2}$ 02), 4.421(83)(112, 004, 202), 3.781(38)($\bar{2}$
02), 4.421(83)(112, 004, 202), 3.781(38)($\bar{2}$ 12) and 3.586(57)(014, $\bar{2}$
12) and 3.586(57)(014, $\bar{2}$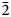 04). Uranoclite is monoclinic, P21/n, a = 10.763(8), b = 6.156(8), c = 17.798(8) Å, β = 95.656(15)°, V = 1173.5(18) Å3 and Z = 4. The structure is the same as that of synthetic (UO2)2(OH)2Cl2(H2O)4 in which the structural unit is a dimer consisting of two pentagonal bipyramids that share an equatorial OH–OH edge. The dimers are linked to one another only by hydrogen bonding. This is the second known uranyl mineral containing essential Cl and the first in which Cl coordinates to U6+.
04). Uranoclite is monoclinic, P21/n, a = 10.763(8), b = 6.156(8), c = 17.798(8) Å, β = 95.656(15)°, V = 1173.5(18) Å3 and Z = 4. The structure is the same as that of synthetic (UO2)2(OH)2Cl2(H2O)4 in which the structural unit is a dimer consisting of two pentagonal bipyramids that share an equatorial OH–OH edge. The dimers are linked to one another only by hydrogen bonding. This is the second known uranyl mineral containing essential Cl and the first in which Cl coordinates to U6+.
Associate Editor: Sergey V Krivovichev