Article contents
Trigonal variation in the garnet supergroup: the crystal structure of nikmelnikovite, Ca12Fe2+Fe3+3Al3(SiO4)6(OH)20, from Kovdor massif, Kola Peninsula, Russia
Published online by Cambridge University Press: 28 June 2021
Abstract
The crystal structure of nikmelnikovite, Ca12Fe2+Fe3+3Al3(SiO4)6(OH)20, a new member of the garnet supergroup from Kovdor massif, Kola Peninsula, Russia (R$\bar{3}$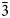 , a = 17.2072(6), c = 10.5689(4) Å, V = 2710.1(2) Å3 and Z = 3) has been refined to R1 = 0.046 on the basis of 1184 unique observed reflections. Nikmelnikovite is the first mineral species in the garnet supergroup that has a trigonal (rhombohedral) symmetry. The relationship between its unit cell and the pseudocubic (ideal garnet) unit cell can be described by the transformation matrix [1$\bar{1}$
, a = 17.2072(6), c = 10.5689(4) Å, V = 2710.1(2) Å3 and Z = 3) has been refined to R1 = 0.046 on the basis of 1184 unique observed reflections. Nikmelnikovite is the first mineral species in the garnet supergroup that has a trigonal (rhombohedral) symmetry. The relationship between its unit cell and the pseudocubic (ideal garnet) unit cell can be described by the transformation matrix [1$\bar{1}$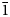 0 | 01$\bar{1}$
0 | 01$\bar{1}$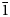 | ½½½]. The crystal-chemical relations between the ideal Ia$\bar{3}$
| ½½½]. The crystal-chemical relations between the ideal Ia$\bar{3}$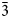 d garnet and the nikmelnikovite structure type can be described by the following series of imaginary modifications: (1) the symmetry is lowered according to the Ia$\bar{3}$
d garnet and the nikmelnikovite structure type can be described by the following series of imaginary modifications: (1) the symmetry is lowered according to the Ia$\bar{3}$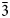 d → R$\bar{3}$
d → R$\bar{3}$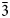 group–subgroup relationship; (2) the cation sites are split according to the following sequences: X → {X1, X2}; Y → {Y1, Y2, Y3, Y4}; Z → {Z1, Z2}; (3) the X sites remain fully occupied by Ca; (4) each Y site is occupied predominantly by a distinct chemical species: Y1 → Al (Al site), Y2 → Fe2+ (Fe1 site), Y3 → Fe3+ (Fe2 site), Y4 → vacancy (Mn site); (5) one of the Z sites (Z1) is occupied by Si, whereas the other site (Z2) is predominantly vacant. The crystal-chemical formula that takes into account the transition between the archetype and the nikmelnikovite structure type can be described as X{Ca12}Y[Fe2+Al4Fe3+2□]Z(Si6□6)O24(OH)20□4. The structural complexity of nikmelnikovite (4.529 bit/atom and 434.431 bit/cell, after H-correction) is higher than those for andradite, grossular and katoite, which is typical for low-temperature minerals formed after primary minerals with simpler structures.
group–subgroup relationship; (2) the cation sites are split according to the following sequences: X → {X1, X2}; Y → {Y1, Y2, Y3, Y4}; Z → {Z1, Z2}; (3) the X sites remain fully occupied by Ca; (4) each Y site is occupied predominantly by a distinct chemical species: Y1 → Al (Al site), Y2 → Fe2+ (Fe1 site), Y3 → Fe3+ (Fe2 site), Y4 → vacancy (Mn site); (5) one of the Z sites (Z1) is occupied by Si, whereas the other site (Z2) is predominantly vacant. The crystal-chemical formula that takes into account the transition between the archetype and the nikmelnikovite structure type can be described as X{Ca12}Y[Fe2+Al4Fe3+2□]Z(Si6□6)O24(OH)20□4. The structural complexity of nikmelnikovite (4.529 bit/atom and 434.431 bit/cell, after H-correction) is higher than those for andradite, grossular and katoite, which is typical for low-temperature minerals formed after primary minerals with simpler structures.
- Type
- Article – Gregory Yu. Ivanyuk memorial issue
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Deceased
Associate Editor: Juraj Majzlan
References
- 7
- Cited by


