Introduction
Mercury (Hg, Z = 80) is a relatively rare element in the Upper Crust (~0.05 μg/g; Rudnick and Gao, Reference Rudnick, Gao and Rudnick2004). The multifaceted Hg mineralogy, with more than 100 mineral species having it as a fundamental chemical constituent, is related to the complex crystal chemistry of this element. Indeed, Hg occurs in three different oxidation states (Hg0, Hg+ and Hg2+) and displays different coordinations with anions (from linear to eight-fold); moreover, it can also form Hg clusters (Hazen et al., Reference Hazen, Golden, Downs, Hystad, Grew, Azzolini and Sverjensky2020). Mercury is typically a chalcophile element (indeed more than half of its minerals can be classified as sulfides and sulfosalts) but is able to form bonds with O2–, halogens, as well as complex anions [e.g. (SO4)2–, (CO3)2–, (SiO4)4–…]. This versatility is probably the reason for its relatively high ‘diversity index’ (D = 4.47; Christy, Reference Christy2015), i.e. the number of its known species is larger than that predicted on the basis of its crustal abundance.
In addition, mercury can occur as a minor constituent in several chalcogenides, e.g. sphalerite (e.g. Dini et al., Reference Dini, Benvenuti, Lattanzi and Tanelli1995; Grammatikopoulos et al., Reference Grammatikopoulos, Valeyev and Roth2006; Çiftçi, Reference Çiftçi2009), tetrahedrite-group minerals (e.g. Arlt and Diamond, Reference Arlt and Diamond1998; Foit and Ulbricht, Reference Foit and Ulbricht2001; Velebil, Reference Velebil2014), and in more complex sulfosalts (e.g. izoklakeite – Orlandi et al., Reference Orlandi, Moëlo and Biagioni2010; sterryite – Moëlo et al., Reference Moëlo, Orlandi, Guillot-Deudon, Biagioni, Paar and Evain2011). Concerning the tetrahedrite-group minerals, Hg can be so abundant to be considered an essential constituent, being the dominant C cation in the general formula M (2)A6M (1)(B4C2)X (3)D4S(1)Y12S(2)Z (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a). Currently, three mineral species belonging to this group have Hg as the dominant C constituent, i.e. hakite-(Hg) (Johan and Kvaček, Reference Johan and Kvaček1971), tetrahedrite-(Hg) (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b) and argentotetrahedrite-(Hg) (Wu et al., Reference Wu, Gu, Qu, Yang and Wang2021). Pošepnýite, ideally (Cu3□3)(Cu2Hg4)Sb4Se12(Se0.5□0.5) (Škácha et al., Reference Škácha, Sejkora, Plášil and Makovicky2020) is another Hg-rich species belonging to the tetrahedrite group.
During the systematic investigation of specimens from the Lengenbach quarry, Binn Valley, Switzerland, one of us (PR) examined a sample belonging to another of us (TR) who bought it in 2016 from the mineral dealer Toni Imhof, in Binn. Preliminary chemical analyses suggested that the sample studied could be a potential candidate for the description of the new mineral tennantite-(Hg). The subsequent crystal chemical study confirmed this first hypothesis and tennantite-(Hg) (as well as its name, following the nomenclature of tetrahedrite-group minerals – Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a) was approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA–CNMNC) under the IMA number 2020-063 (Biagioni et al., Reference Biagioni, Sejkora, Raber, Roth, Moëlo, Dolníček and Pasero2020c). Holotype material is deposited in the mineralogical collections of the Museo di Storia Naturale, Università di Pisa, Via Roma 79, Calci (Pisa), Italy, catalogue number 19919.
In this paper the description of tennantite-(Hg) is reported.
Occurrence and physical properties
Tennantite-(Hg) was found in the Lengenbach quarry, Imfeld, Binn Valley, Canton Valais, Switzerland (46°21’54”N, 8°13’15”E). The quarry exploits part of a 240-m thick Triassic metadolostone occurring at the front of the Monte Leone Nappe, in the Penninic domain of the Alps; the mineralisation occurs in the uppermost part of the metadolostone, 180 to 200 m above its base, close to the contact with Jurassic to Lower Cretaceous ‘Bündnerschiefer’. According to Hofmann (Reference Hofmann1994), the Lengenbach deposit may be the result of the metamorphic recrystallisation and remobilisation, through hydrothermal fluids as well as sulfide melts, of a pre-existing carbonate-hosted Pb–Zn–Ag–Cu–Cd–Tl–As ore deposit that experienced P–T conditions up to the upper greenschist – lower amphibolite facies. During the late stage of the evolution of the Lengenbach deposit, the enrichment of Tl and As (and probably of Hg) occurred, resulting in the crystallisation of a series of Tl sulfarsenites and realgar. In agreement with Graeser et al. (Reference Graeser, Cannon, Drechsler, Raber and Roth2008), Tl- and As-enriched ores can be usually found in the so-called ‘Zone 1’, one of the five major bedding-parallel zones that can be distinguished in the quarry on the basis of the observed mineral assemblages. Tennantite-(Hg) was probably collected in this realgar-bearing ‘Zone 1’. A review of the mineralogy of the Lengenbach quarry is given in Roth et al. (Reference Roth, Raber, Drechsler and Cannon2014).
Tennantite-(Hg) was identified in only one sample as an aggregate of tetrahedral crystals, less than 0.1 mm in size (Fig. 1). Colour and streak are black, the lustre is metallic. Mohs hardness was not measured owing to the scarcity of available material and its small size; it may be close to 3½–4, in agreement with the hardness of other members of the tetrahedrite group. Tennantite-(Hg) is brittle, with an indistinct cleavage and a conchoidal fracture. Density was not measured, owing to the small amount of available material. Calculated density, on the basis of the empirical formula and single-crystal unit-cell parameters, is 4.838 g/cm3.
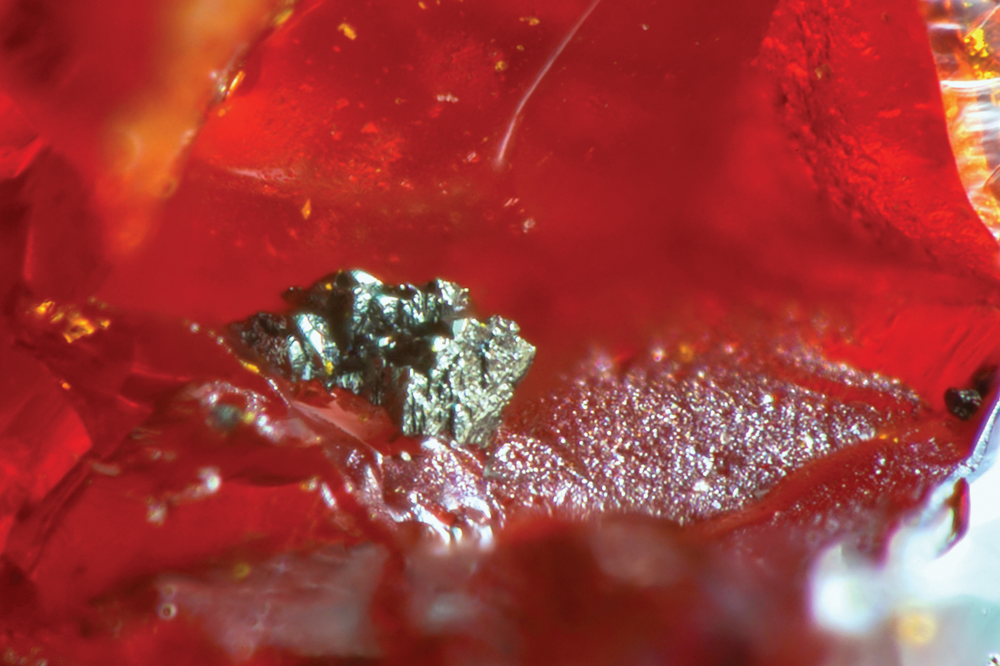
Fig. 1. Tennantite-(Hg), tetrahedral crystals up to 0.1 mm, with realgar. Lengenbach, Binn Valley, Switzerland. Collection T. Raber, photo M. Crumbach.
In reflected light, tennantite-(Hg) is isotropic, showing a grey colour with creamy tint. Internal reflections were not observed. Reflectance values, measured in air with a MSP400 Tidas spectrophotometer and a Leica microscope with a 100× objective, are reported in Table 1 and shown in Fig. 2.

Fig. 2. Reflectance curves for tennantite-(Hg) in air. For comparison, the reflectance curves of tetrahedrite-(Hg) from Buca della Vena (Italy), Jedová hora (Czech Republic), and Rožňava (Slovakia) (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b) are shown.
Table 1. Reflectance data for tennantite-(Hg).*

* The reference wavelengths required by the Commission on Ore Mineralogy (COM) are given in bold.
Tennantite-(Hg) is associated with realgar and is intimately intergrown with a Cu–As–S phase, probably represented by sinnerite, having minor Ag (ca. 1.5 wt.%) and Hg (ca. 2.5 wt.%). The crystallisation of tennantite-(Hg) is probably related to the late-stage circulation of hydrothermal fluids within the Lengenbach metadolostone.
Chemical data
Quantitative chemical analyses were carried out using a Cameca SX 100 electron microprobe (National Museum, Prague, Czech Republic) and the following experimental conditions: wavelength-dispersive mode, accelerating voltage 25 kV, beam current 20 nA, beam diameter 1 μm. Standards (element, emission line) were: chalcopyrite (CuKα and SKα), ZnS (ZnKα), NiAs (AsLβ), Ag metal (AgLα), Sb2S3 (SbLα), HgTe (HgMα), TlBr (TlMα), and PbS (PbMα). Matrix correction by PAP software (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985) was applied to the data. Results (average of 7 spot analyses) are given in Table 2. The amount of other elements with Z > 8 (including Fe) was below detection limits.
The empirical formula of tennantite-(Hg), recalculated on the basis of ΣMe = 16 atoms per formula unit, is (with rounding errors) (Cu4.69Ag1.04Tl0.03)Σ5.76(Cu4.35Hg1.58Pb0.06Zn0.01)Σ6.00(As4.20Sb0.05)Σ4.25S13.26.
The end-member formula of tennantite-(Hg) is Cu6(Cu4Hg2)As4S13 (Z = 2), corresponding to (in wt.%) Cu 36.25, Hg 22.88, As 17.09, S 23.78, total 100.00.
X-ray crystallography
Single-crystal X-ray diffraction intensity data were collected on tennantite-(Hg) using a Bruker Smart Breeze diffractometer (50 kV, 30 mA) equipped with a Photon II CCD detector and graphite-monochromatised MoKα radiation (Dipartimento di Scienze della Terra, Università di Pisa). The detector-to-crystal distance was set at 50 mm. Data were collected using ω scan mode in 0.5° slices, with an exposure time of 90 s per frame, and corrected for Lorentz and polarisation factors as well as for absorption using the software package Apex3 (Bruker AXS Inc., 2016). The refined unit-cell parameter is a = 10.455(7) Å and V = 1143(2) Å3 in space group I $\overline 4$![]() 3m. The crystal structure of tennantite-(Hg) was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the structural model of tetrahedrite-(Hg) given by Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b). The following neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992), were used: Cu vs. Ag at M(2); Cu vs. Hg at M(1); As vs. Sb at X(3); and S at S(1) and S(2) sites. After several cycles of isotropic refinement, the agreement factor R 1 converged to 0.123, thus confirming the correctness of the structural model. Residuals in the difference-Fourier maps suggested the split nature of the M(2) site, in agreement with the finding of previous investigators (e.g. Andreasen et al., Reference Andreasen, Makovicky, Lebech and Karup-Møller2008; Welch et al., Reference Welch, Stanley, Spratt and Mills2018). The splitting of the M(2) site into two positions, M(2a) at (0, 0, z) and M(2b) at (x, x, z) lowered the R 1 value to 0.105; the site scattering at these sub-sites was modelled using the scattering factors of Cu and Ag at the M(2a) and M(2b) positions, respectively. At this stage of the refinement, the modelling of the racemic twin suggested that the structure should be inverted. Moreover, in order to have a good data/parameter ratio, the site occupancy factor (s.o.f.) at the M(1) site was fixed. The refined s.o.f., Cu0.67(3)Hg0.33(3), corresponds to 45.83 electrons per site, to be compared with the site population Cu0.73Hg0.27 idealised from the chemical data (see below) and corresponding to 42.77 electrons per site. The s.o.f. factor at the M(1) was then fixed at the value Cu0.70Hg0.30, i.e. 44.3 electrons per site, an average between refined s.o.f. and chemical data. Through the anisotropic modelling of the displacement parameters for cations, the refinement converged to R 1 = 0.098. Finally, the anisotropic structural model for all atoms converged to R 1 = 0.0897 for 214 reflections with F o > 4σ(F o) and 22 refined parameters. Details of data collection and refinement are given in Table 3. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 4. Table 5 reports selected bond distances. Finally, Table 6 gives the results of the bond-valence calculations obtained using the bond-valence parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991). The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
3m. The crystal structure of tennantite-(Hg) was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the structural model of tetrahedrite-(Hg) given by Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b). The following neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992), were used: Cu vs. Ag at M(2); Cu vs. Hg at M(1); As vs. Sb at X(3); and S at S(1) and S(2) sites. After several cycles of isotropic refinement, the agreement factor R 1 converged to 0.123, thus confirming the correctness of the structural model. Residuals in the difference-Fourier maps suggested the split nature of the M(2) site, in agreement with the finding of previous investigators (e.g. Andreasen et al., Reference Andreasen, Makovicky, Lebech and Karup-Møller2008; Welch et al., Reference Welch, Stanley, Spratt and Mills2018). The splitting of the M(2) site into two positions, M(2a) at (0, 0, z) and M(2b) at (x, x, z) lowered the R 1 value to 0.105; the site scattering at these sub-sites was modelled using the scattering factors of Cu and Ag at the M(2a) and M(2b) positions, respectively. At this stage of the refinement, the modelling of the racemic twin suggested that the structure should be inverted. Moreover, in order to have a good data/parameter ratio, the site occupancy factor (s.o.f.) at the M(1) site was fixed. The refined s.o.f., Cu0.67(3)Hg0.33(3), corresponds to 45.83 electrons per site, to be compared with the site population Cu0.73Hg0.27 idealised from the chemical data (see below) and corresponding to 42.77 electrons per site. The s.o.f. factor at the M(1) was then fixed at the value Cu0.70Hg0.30, i.e. 44.3 electrons per site, an average between refined s.o.f. and chemical data. Through the anisotropic modelling of the displacement parameters for cations, the refinement converged to R 1 = 0.098. Finally, the anisotropic structural model for all atoms converged to R 1 = 0.0897 for 214 reflections with F o > 4σ(F o) and 22 refined parameters. Details of data collection and refinement are given in Table 3. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 4. Table 5 reports selected bond distances. Finally, Table 6 gives the results of the bond-valence calculations obtained using the bond-valence parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991). The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 3. Summary of crystal data and parameters describing data collection and refinement for tennantite-(Hg).

* w = 1/[σ2(F o2)+(0.1032P)2+23.0364P], where P = (F o2+2F c2)/3
** Flack (Reference Flack1983)
Table 4. Sites, Wyckoff positions, site occupancy factors (s.o.f.), fractional atom coordinates and equivalent isotropic displacement parameters (Å2) for tennantite-(Hg).

Table 5. Selected bond distances (Å) for tennantite-(Hg).

Table 6. Weighted bond-valence sums (in valence unit) for tennantite-(Hg).*

* Note: left and right superscripts indicate the number of equivalent bonds involving cations and anions, respectively. The following site populations were used: M(2a) = Cu0.81; M(2b) = Ag0.095; M(1) = Cu0.70Hg0.30; X(3) = As0.93Sb0.07.
Owing to the small size of the available crystal of tennantite-(Hg), powder X-ray diffraction data were not collected. The calculated pattern, based on the structural model given in Table 4, is reported in Table 7.
Table 7. Calculated powder X-ray diffraction data for tennantite-(Hg).*

* Intensity and d hkl were calculated using the software PowderCell2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) on the basis of the structural model given in Table 4. Only reflections with I calc > 1 are listed. The six strongest reflections are given in bold.
Results and discussion
Crystal structure description
The crystal structure of tennantite-(Hg) is isotypic with those of the other members of the tetrahedrite group and it can be described as a collapsed sodalite-like framework of corner-sharing M(1)-centred tetrahedra giving rise to cages where S(2)-centred M(2)-octahedra and X(3)S(1)3 trigonal pyramids are hosted (e.g. Johnson et al., Reference Johnson, Craig and Rimstidt1988).
The three-fold coordinated M(2) site is split into two sub-positions, namely M(2a) and M(2b) (Fig. 3). The former has an average <M(2a)–S> bond distance of 2.285 Å, whereas the average <M(2b)–S> distance is larger, i.e. 2.46 Å. Copper is probably located mainly at the smaller M(2a) position, and the Ag+ cation may be hosted preferentially at the larger M(2b). The site scattering refined at the M(2a)+M(2b) site is ~194.5 electrons per formula unit (epfu), corresponding to the idealised population (Cu4.9Ag1.1), in quite good agreement with chemical data. Electron microprobe analysis led to the site population M (2)(Cu4.69Ag1.04Tl0.03□0.24), corresponding to ~187.3 epfu. Whereas the occurrence of vacancies at M(2) is known in some tetrahedrite-group minerals (e.g. goldfieldite – Makovicky and Karup-Møller, Reference Makovicky and Karup-Møller2017; pošepnýite – Škácha et al., Reference Škácha, Sejkora, Plášil and Makovicky2020), it is likely that the deficit of M(2) cations observed in tennantite-(Hg) could be an analytical artefact. Indeed, normalising the observed site population to 6 atoms per formula unit (apfu), one obtains M (2)(Cu4.89Ag1.08Tl0.03), corresponding to a calculated site scattering of 195 epfu, in good accord with structural data. Thallium was grouped along with other formally monovalent cations (Cu, Ag), although it is more likely that it could replace S at the S(2) position, assuming a configuration similar to that observed in routhierite-group or in galkhaite-group minerals (e.g. Borisov et al., Reference Borisov, Magarill and Pervukhina2009; Bindi and Biagioni, Reference Bindi and Biagioni2018; Makovicky, Reference Makovicky2018). Indeed, both the substitution mechanisms 6M (2)Me+ + 2M (1)Me+ + S2– = 6M (2)□ + 2M (1)Me2+ + 2Tl+ (tetrahedrite-to-routhierite substitution) and 6M (2)Me+ + 3M (1)Me+ + S2- = 6M (2)□ + 3M (1)Hg2+ + (Tl/Cs)+ (tetrahedrite-to-galkhaite substitution) can be hypothesised. Assuming the site population (Cu4.9Ag1.1), the bond-valence sum (BVS) at M(2a) + 2×M(2b) is 1.06 valence units (vu), in agreement with the occurrence of monovalent cations.

Fig. 3. M(2a) and M(2b) split sites, hosting Cu and Ag respectively, in S(1) four-capped truncated tetrahedra, around the central S(2) atom.
The tetrahedrally coordinated M(1) site (Fig. 4) has an average <M(1)–S> bond distance of 2.389 Å, to be compared with 2.391 Å observed in tetrahedrite-(Hg) with similar Hg content (1.64 apfu – Biagioni et al., Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b). Chemical data indicate the occurrence of Cu and Hg, with only minor substitution of Pb and Zn. Lead was attributed to the M(1) site, in agreement with Vavelidis and Melfos (Reference Vavelidis and Melfos1997) who reported the occurrence of a potentially new tetrahedrite-group mineral having Pb dominant over Fe and Zn from the Maronia area, Greece. However, Makovicky and Karup-Møller (Reference Makovicky and Karup-Møller1994) suggested that the presence of very fine exsolutions of Pb-rich phases cannot be excluded to explain the Pb content. Consequently, the actual speciation of Pb is currently unclear. Copper probably also occurs as a formally divalent cation; its content was calculated in order to achieve 2Me 2+ apfu. Ignoring minor Pb and Zn, the site population at M(1) could be [Cu+4(Hg1.6Cu2+0.4)]. On the basis of this site population and using the atomic radii proposed by Johnson et al. (Reference Johnson, Craig and Rimstidt1988), the calculated M(1)–S(1) bond distance should be 2.390 Å, matching the observed value. The bond-valence sum at M(1) is 1.68 vu, higher than the theoretical value of 1.33 vu, and similar to previous results on sulfosalts having tetrahedrally coordinated Hg (e.g. arsiccioite, AgHg2TlAs2S6 – Biagioni et al., Reference Biagioni, Bonaccorsi, Moëlo, Orlandi, Bindi, D'Orazio and Vezzoni2014); this could be possibly due to the inaccuracy of the bond valence parameter for the Hg–S pair.
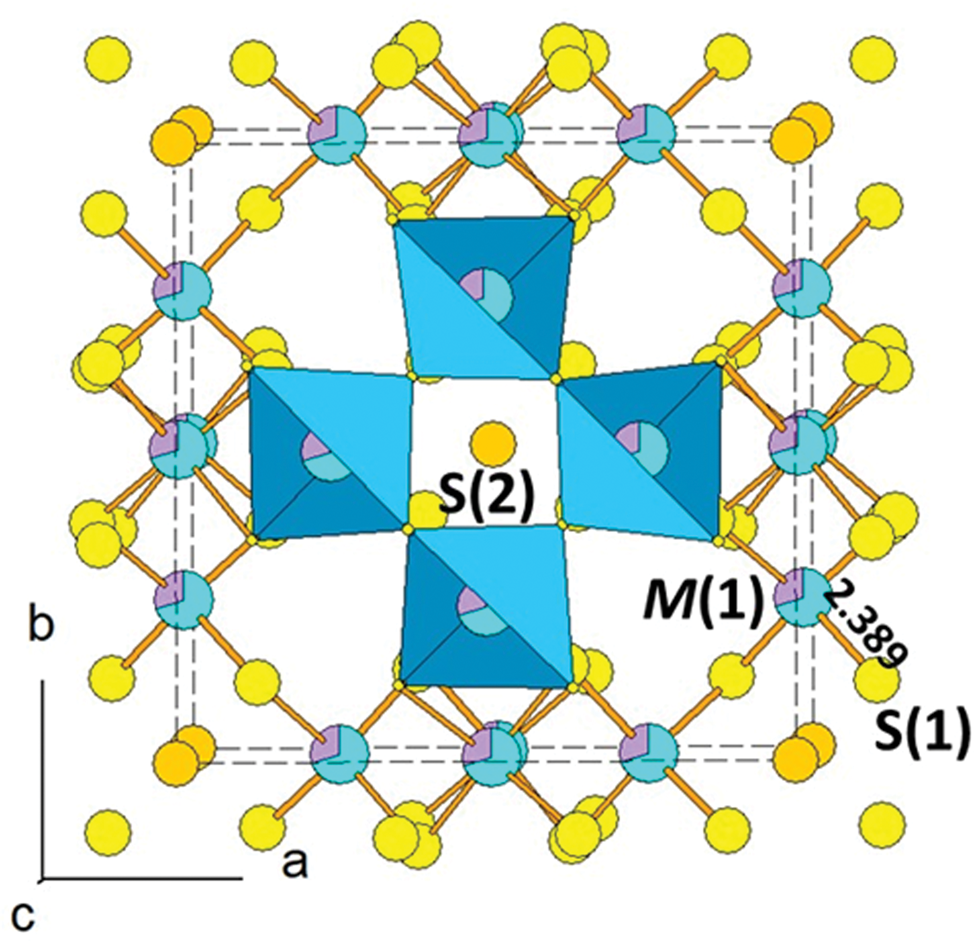
Fig. 4. The mixed (Cu,Hg) M(1) site, with regular tetrahedral coordination, in the crystal structure of tennantite-(Hg).
The X(3) site has an average bond distance of 2.280 Å, agreeing with the As-dominant nature of this position (Fig. 5). Taking into account the ideal As–S and Sb–S distances (2.26 and 2.45 Å, respectively, calculated from the bond parameters of Brese and O'Keeffe, Reference Brese and O'Keeffe1991), such an observed distance would correspond to the site population (As0.89Sb0.11), not far from the refined value (As0.93Sb0.07). On the contrary, electron microprobe analysis indicate only very minor Sb content, and an excess of As, with a sum (As+Sb) close to 4.25 apfu. The bond-valence sum, calculated assuming the site population (As0.98Sb0.02), in keeping with chemical data, is 2.88 vu; assuming the refined site occupancy (As0.93Sb0.07), the BVS is 2.97 vu.

Fig. 5. Trigonal X(3)(S1)3 pyramids in the unit cell of tennantite-(Hg).
The S(1) site is four-fold coordinated, being bonded to two M(1), one M(2), and one X(3). Its BVS is 2.17 vu. S(2) is octahedrally coordinated by atoms hosted at M(2) sites, with a BVS of 2.28 vu. No vacancies were observed at S(2) during the structure refinement.
Taking into account the results of the crystal structure refinement, the structural formula M (2)(Cu4.9Ag1.1)M (1)[Cu4(Hg1.6Cu2+0.4)]X (3)(As3.7Sb0.3)S13 can be proposed. Using this formula, the unit-cell parameter can be calculated using the relations proposed by Johnson et al. (Reference Johnson, Craig and Rimstidt1987) (corrected according to Di Benedetto et al., Reference Di Benedetto, Bernardini, Borrini, Emiliani, Cipriani, Danti, Caneschi, Gatteschi and Romanelli2002); the calculated value is a = 10.42 Å, to be compared with the measured one, i.e. a = 10.455 Å. The difference between calculated and observed values can be compared with those reported by Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b) for three samples of tetrahedrite-(Hg). The increase of the a parameter relative to tennantite-(Zn) (10.232 Å – Wuensch et al., Reference Wuensch, Takéuchi and Nowacki1966) is related to the Hg content, in agreement with the study of Karup-Møller and Makovicky (Reference Karup-Møller and Makovicky2003), as well as to the presence of 1.1 Ag apfu.
It may be interesting to compare some structural details of tennantite-(Hg) with those shown by tetrahedrite-(Hg) (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b). The former shows a smaller framework rotation (as defined by Johnson et al., Reference Johnson, Craig and Rimstidt1988) than that observed in tetrahedrite-(Hg), i.e. 50.1° vs. 51.3°. Whereas the rotation angle of tetrahedrite-(Hg) is in keeping with the value estimated on the basis of the M(1)–S(1) bond distance using figure 8a of Johnson et al. (Reference Johnson, Craig and Rimstidt1988), the corresponding value for tennantite-(Hg) is slightly smaller than the expected one. This could be explained by the partial occurrence of Ag at the M(2) site that favours a lengthening of the spinner-blade length (as defined by Johnson et al., Reference Johnson, Craig and Rimstidt1988) with respect to Ag-free tennantite-series minerals, thus causing a decrease in the amount of rotation. With As replacing for Sb, the S(1)–M(2)–S(1) angle [considering the M(2a) position for tennantite-(Hg)] increases from 96.5(2)° to 103.5(8)°; moreover, the X(3)S(1)3 trigonal pyramids become more flattened, with S(1)–X(3)–S(1) angles varying from 95.24(10)° in tetrahedrite-(Hg) to 99.1(4)° in tennantite-(Hg). These changes are required to allow a better match between the smaller AsS3 pyramids with the spinner-blade and the tetrahedral framework characterising tetrahedrite-group minerals.
Other findings of tennantite-(Hg)
The possible presence of Hg in tetrahedrite-group minerals has been known since the 19th Century. Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b) briefly reviewed the occurrences of tetrahedrite-(Hg), showing that several findings of Hg-dominant or Hg-bearing tetrahedrite-series minerals are reported worldwide. Usually, Hg-rich tennantite-series minerals are quite rare, as suggested by Johnson et al. (Reference Johnson, Craig and Rimstidt1986), who observed how Hg-rich tetrahedrite-group minerals tend to be Sb-rich. This was confirmed by Foit and Ulbricht (Reference Foit and Ulbricht2001) who studied the compositional variation of Hg-bearing tetrahedrite/tennantite from the Steens and Pueblo Mountains, Oregon, USA.
To the best of our knowledge, one of the first reports of Hg-bearing tennantite was given by Faick (Reference Faick1958) from the Ord mine, Gila County, Arizona, USA. This mineral occurs as small grains intergrown with chalcocite in a gangue formed by quartz, Mg-bearing siderite, and minor baryte. The critical examination of chemical data suggested that the studied ore sample contains minor Mg-bearing siderite, as suggested by the occurrence of 1.1 wt.% MgO. Consequently, the formula was calculated assuming that all Fe was due to the occurrence of such a carbonate. The formula, based on ΣMe = 16 apfu, is (with rounding errors) Cu6.16(Cu4.26Hg1.74)Σ6.00(As2.67Sb1.16)Σ3.83S12.99, thus supporting its identification as tennantite-(Hg). Samples corresponding to tennantite-(Hg) were later reported by Mozgova et al. (Reference Mozgova, Tsepin and Ozerova1978) and Mozgova and Tsepin (Reference Mozgova and Tsepin1983). Mozgova et al. (Reference Mozgova, Tsepin and Ozerova1978) described this mineral from the ore deposit of Kulponei, Chukotka Autonomous Okrug (Russia), with chemical formula Cu5.68(Cu4.11Hg1.82Zn0.05Fe0.02)(As3.90Sb0.10)S13.07 and unit-cell parameter a = 10.34 Å. This composition is very close to the ideal end-member Cu6(Cu4Hg2)As4S13. Pervukhina et al. (Reference Pervukhina, Borisov, Magarill, Vasuliev, Kuratieva and Kozlova2010) described the crystal structure of ‘As-schwatzite’ from Aktash, Altai Republic, Russia; actually, the chemical formula of their sample, recalculated on the basis of ΣMe = 16 apfu, is Cu6.24(Cu4.65Fe0.73Hg0.62)Σ6.00(As2.97Sb0.79)Σ3.76S12.64, with Fe > Hg, indicating that the material studied was likely to be a Hg-rich tennantite-(Fe). Steed (Reference Steed1983) cited the presence of ‘mercurian tennantite’ in the Gortdrum orebody, County Tipperary, Ireland, without providing any data.
Following the approval of tennantite-(Hg) from the Lengenbach quarry, two other occurrences have been reported. Wei et al. (Reference Wei, Xia, Steadman, Xie, Liu, Tan and Bai2021) described tennantite-(Hg) (Hg up to 1.34 apfu), as μm-sized (~1–2 μm) grains associated with tennantite-(Zn), from the Nibao Carlin-type Au deposit, Guizhou, China, whereas Ansermet et al. (Reference Ansermet, Cuchet, Roth and Meisser2021) reported tennantite-(Hg) from the Chandolin Alp, Valais, Switzerland, as a 70 μm anhedral grain associated with gold, bismuth, covellite, hessite, spionkopite and chalcocite, all included in bornite. On the basis of energy-dispersive spectroscopy analyses, the chemical formula, recalculated assuming ΣMe = 16 apfu, is Cu9.62Hg2.02(As4.05Sb0.31)Σ4.36S13.37 (N. Meisser, pers. comm.).
The finding of tennantite-(Hg) and the re-examination of previous occurrences suggest that further data could indicate a wide solid solution between this phase and its Sb counterpart, as observed in the Se isotypes hakite-(Hg), Cu6(Cu4Hg2)Sb4Se13, and the potential new end-member giraudite-(Hg), Cu6(Cu4Hg2)As4Se13 (e.g. Förster et al., Reference Förster, Rhede and Tischendorf2002).
Mercury minerals in the Lengenbach quarry: a review
The Lengenbach quarry is well-known worldwide for its sulfosalt assemblages, typically characterised by the presence of Pb–As–Tl–Ag–Cu (e.g. Roth et al., Reference Roth, Raber, Drechsler and Cannon2014; Raber and Roth, Reference Raber and Roth2018). Hofmann and Knill (Reference Hofmann and Knill1996) reported a 100- to 1000-fold higher content of Hg in the mineralised metadolostone than in the unmineralised one, similar to the enrichment factors of some other elements, e.g. Ag, Tl and Zn. However, such a geochemical Hg-enrichment has a comparatively discrete mineralogical expression only. Indeed, Hg minerals are rare in the Lengenbach quarry and, with the exception of cinnabar, were found in the Tl- and As-rich ‘Zone 1’ only.
The first new Hg-mineral described from the Lengenbach quarry was debattistiite, Ag9Hg0.5As6S12Te2 (Guastoni et al., Reference Guastoni, Bindi and Nestola2012). In this phase, Hg occurs in a partially occupied site and shows a linear coordination with two Te atoms at 2.594 Å. A similar linear coordination can be observed in cinnabar; this sulfide was found only once as a reddish crust grown on a rounded crystal of tennantite-(Zn) (no Hg was detected in that sample of tennantite through energy-dispersive spectroscopy analysis) (Graeser et al., Reference Graeser, Cannon, Drechsler, Raber and Roth2008).
The linear coordination is quite common for Hg, whereas the other main kind of coordination, tetrahedral, is rarer. In addition to tennantite-(Hg), currently found in only one specimen from the Lengenbach quarry, other minerals identified at this locality and characterised by tetrahedrally-coordinated Hg are aktashite, coloradoite and routhierite. All these species are very rare. Aktashite, Cu6Hg3As4S12, was found in grey porous aggregates and pseudo-cubic crystals, associated with realgar; Hg has been detected also in its Zn-isotype nowackiite (Roth and Raber, Reference Roth and Raber2018). Coloradoite was reported in close association with sinnerite by Bindi et al. (Reference Bindi, Makovicky, Nestola and De Battisti2013); it is worth noting that sinnerite, intimately intergrown with tennantite-(Hg), contains minor Hg. Indeed, sinnerite shows 12 independent tetrahedrally-coordinated Cu sites, potentially able to host Hg; moreover, its relationships with the sphalerite structure, first pointed out by Makovicky and Skinner (Reference Makovicky and Skinner1972), could explain the intergrowth between this mineral and tennantite-(Hg). Minor Hg was also detected in Te-rich canfieldite from the ‘Zone 1’ of the Lengenbach quarry (Raber and Roth, Reference Raber and Roth2014).
At the Lengenbach quarry the main carriers of Hg are likely to be the members of the routhierite isotypic series. In addition to routhierite, CuHg2TlAs2S6, only recently formally identified at this locality (Graeser et al., Reference Graeser, Cannon, Drechsler, Raber and Roth2008; Roth, Reference Roth2016), Hg also occurs in detectable amounts in stalderite (8.90 wt.% – Graeser et al., Reference Graeser, Schwander, Wulf and Edenharter1995), ralphcannonite (7.92 wt.% – Bindi et al., Reference Bindi, Biagioni, Raber, Roth and Nestola2015), and ferrostalderite (1.22 wt.% – Biagioni et al., Reference Biagioni, Bindi, Nestola, Cannon, Roth and Raber2016). It is interesting to observe that the majority of the identified specimens of routhierite-group minerals collected at the Lengenbach quarry were found as epitactic overgrowths on tennantite-(Zn). Considering the structural relationships between tetrahedrite- and routhierite-group minerals, an increase in the Tl content in the late-stage hydrothermal fluids could favour the stabilisation of routhierite-group minerals with respect to tetrahedrite-group phases, as suggested by Biagioni et al. (Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b). If so, we could hypothesise that tennantite-(Hg) formed in a locally Tl-depleted environment; in this way, Hg occurring in the fluids was incorporated in the crystal structure of this tetrahedrite-group mineral.
Conclusion
The finding and description of the new tetrahedrite-group mineral tennantite-(Hg) add further data on the crystal chemistry of this important group of ore minerals, suggesting that peculiar crystallisation conditions may allow the crystallisation of (Hg/As)-rich members. This seems to be the case in the Lengenbach deposit, where the Hg- and As-rich geochemistry, coupled with the usual Tl abundance, favours the crystallisation of routhierite-group minerals; however, the extreme variability of natural hydrothermal systems can lead to the formation of local micro-environments characterised by peculiar physico-chemical conditions promoting the crystallisation of minerals showing unusual associations of elements. This could be the case of tennantite-(Hg) from the Lengenbach quarry, which could have formed as a result of a very localised Tl-depletion. This again underlines the importance of studies performed on natural ore assemblages for a full understanding of the crystal chemistry of chalcogenides and the pivotal role played in this respect by meticulous mineralogical investigations of the most outstanding natural occurrences like the one represented by the Lengenbach ores.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.59
Acknowledgements
N. Meisser is thanked for additional details about the finding of tennantite-(Hg) from the Chandolin Alp, Switzerland. CB and MP acknowledge financial support from the Ministero dell'Istruzione, dell'Università e della Ricerca through the project PRIN 2017 “TEOREM – deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32. The study was also financially supported by the Ministry of Culture of the Czech Republic (long-term project DKRVO 2019-2023/1.II.c; National Museum, 00023272) for JS and ZD. The comments of Peter Leverett, Xiangping Gu, and an anonymous reviewer improved the original manuscript.
















