Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Filella, Montserrat
2016.
A BUKI (Building up Knowledge Initiative) focussed on antimony’s environmental chemistry.
Environmental Chemistry,
Vol. 13,
Issue. 6,
p.
971.
Majzlan, Juraj
Števko, Martin
and
Lánczos, Tomáš
2016.
Soluble secondary minerals of antimony in Pezinok and Kremnica (Slovakia) and the question of mobility or immobility of antimony in mine waters.
Environmental Chemistry,
Vol. 13,
Issue. 6,
p.
927.
Roper, Adam J.
Leverett, Peter
Murphy, Timothy D.
and
Williams, Peter A.
2018.
The stability of the rare sodium antimonate, brizziite, and it's role in Sb mobility.
Mineralogical Magazine,
Vol. 82,
Issue. 1,
p.
89.
Jarošíková, Alice
Ettler, Vojtěch
Mihaljevič, Martin
Drahota, Petr
Culka, Adam
and
Racek, Martin
2018.
Characterization and pH-dependent environmental stability of arsenic trioxide-containing copper smelter flue dust.
Journal of Environmental Management,
Vol. 209,
Issue. ,
p.
71.
Jarošíková, Alice
Ettler, Vojtěch
Mihaljevič, Martin
Penížek, Vít
Matoušek, Tomáš
Culka, Adam
and
Drahota, Petr
2018.
Transformation of arsenic-rich copper smelter flue dust in contrasting soils: A 2-year field experiment.
Environmental Pollution,
Vol. 237,
Issue. ,
p.
83.
Borčinová Radková, Anežka
Jamieson, Heather E.
and
Campbell, Kate M.
2020.
Antimony mobility during the early stages of stibnite weathering in tailings at the Beaver Brook Sb deposit, Newfoundland.
Applied Geochemistry,
Vol. 115,
Issue. ,
p.
104528.
Ettler, Vojtěch
Mihaljevič, Martin
Jarošíková, Alice
Culka, Adam
Kříbek, Bohdan
Majer, Vladimír
Vaněk, Aleš
Penížek, Vít
Sracek, Ondra
Mapani, Ben
and
Kamona, Fred
2020.
Vanadium-rich slags from the historical processing of Zn–Pb–V ores at Berg Aukas (Namibia): Mineralogy and environmental stability.
Applied Geochemistry,
Vol. 114,
Issue. ,
p.
104473.
Meloni, Federica
Montegrossi, Giordano
Lazzaroni, Marta
Rappuoli, Daniele
Nisi, Barbara
and
Vaselli, Orlando
2021.
Total and Leached Arsenic, Mercury and Antimony in the Mining Waste Dumping Area of Abbadia San Salvatore (Mt. Amiata, Central Italy).
Applied Sciences,
Vol. 11,
Issue. 17,
p.
7893.
Majzlan, Juraj
Kiefer, Stefan
Lilova, Kristina
Subramani, Tamilarasan
Navrotsky, Alexandra
Dachs, Edgar
and
Benisek, Artur
2021.
Chapmanite [Fe<sub>2</sub>Sb(Si<sub>2</sub>O<sub>5</sub>)O<sub>3</sub>(OH)]: thermodynamic properties and formation in low-temperature environments.
European Journal of Mineralogy,
Vol. 33,
Issue. 4,
p.
357.
Radková, A.B.
Jamieson, H.E.
Campbell, K.M.
and
Hudson-Edwards, K.A.
2023.
Antimony in Mine Wastes: Geochemistry, Mineralogy, and Microbiology.
Economic Geology,
Vol. 118,
Issue. 3,
p.
621.
Fancello, Dario
Dore, Elisabetta
Medas, Daniela
Rigonat, Nicola
Meneghini, Carlo
Moroni, Marilena
Naitza, Stefano
Onnis, Patrizia
and
De Giudici, Giovanni
2023.
Antimony contamination sources and alteration pathways of Sb mineral phases in an abandoned mining area: The role of secondary mopungite [NaSb(OH)6].
Applied Geochemistry,
Vol. 156,
Issue. ,
p.
105764.
Juárez-Amador, Lucía Ivonne
Guillén-Bonilla, Héctor
Guillén-Bonilla, Alex
Guillén-Bonilla, José Trinidad
Morales-Bautista, Jacob
Casillas-Zamora, Antonio
Rodríguez-Betancourtt, Verónica-María
and
Olvera-Amador, María de la Luz
2024.
Photocatalytic and sensing properties in propane atmospheres of MgSb2O6 nanoparticles synthesized by a chemical method.
Journal of Materials Science: Materials in Electronics,
Vol. 35,
Issue. 28,
Guillén-Bonilla, Héctor
Guillén-Bonilla, José Trinidad
Rodríguez-Betancourtt, Verónica-María
Ramírez-Ortega, Jorge Alberto
Morán Lázaro, Juan Pablo
and
Guillén-Bonilla, Alex
2024.
Synthesis and Sensing Response of Magnesium Antimoniate Oxide (MgSb2O6) in the Presence of Propane Atmospheres at Different Operating Voltages.
Sensors,
Vol. 24,
Issue. 7,
p.
2147.
Guillén-Bonilla, Alex
Guillén-Bonilla, José Trinidad
Guillén-Bonilla, Héctor
Huízar-Padilla, Emilio
Zamora, Antonio Casillas
de La Luz Olvera Amador, María
and
Rodríguez-Betancourtt, Verónica-María
2024.
A theoretical and experimental study on the dynamic response in propane atmospheres of sensors made from trirutile magnesium antimonate powders.
Journal of Materials Science: Materials in Electronics,
Vol. 35,
Issue. 28,
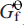 at the same temperature. The derived Δ
at the same temperature. The derived Δ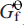 (s, 298.2 K) values for MgSb2O6 (–1554.1 ±3.6 kJ mol–1), ZnSb2O6 (–1257.0 ±2.6 kJ mol–1) and PbSb2O6 (–1154.2 ±2.6 kJ mol–1) have been used in subsequent calculations to determine their relative stabilities and relationships with other secondary Sb minerals.
(s, 298.2 K) values for MgSb2O6 (–1554.1 ±3.6 kJ mol–1), ZnSb2O6 (–1257.0 ±2.6 kJ mol–1) and PbSb2O6 (–1154.2 ±2.6 kJ mol–1) have been used in subsequent calculations to determine their relative stabilities and relationships with other secondary Sb minerals.