Published online by Cambridge University Press: 09 December 2022
The new eudialyte-group mineral selsurtite, ideally (H3O)12Na3(Ca3Mn3)(Na2Fe)Zr3□Si[Si24O69(OH)3](OH)Cl⋅H2O, was discovered in metasomatic peralkaline rock from the Flora mountain, northern spur of the Selsurt mountain, Lovozero alkaline massif, Kola Peninsula, Russia. The associated minerals are aegirine, albite and orthoclase, as well as accessory lorenzenite, calciomurmanite, natrolite, lamprophyllite and sergevanite. Selsurtite occurs as brownish-red to reddish-orange, equant or flattened on (0001) crystals up to 2 mm across and elongate crystals up to 3 cm long. The main crystal forms are {0001}, {11$\bar{2}$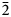 0}, and {10$\bar{1}$
0}, and {10$\bar{1}$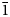 1}. Selsurtite is brittle, with the Mohs’ hardness of 5. No cleavage is observed. Parting is distinct on (001). D(meas) = 2.73(2) and D(calc) = 2.722 g⋅cm–3. Selsurtite is optically uniaxial (–), with ω = 1.598(2) and ɛ = 1.595(2). The chemical composition is (wt.%, electron microprobe): Na2O 6.48, K2O 0.27, MgO 0.10, CaO 6.83, MnO 4.73, FeO 1.18, SrO 1.88, La2O3 0.57, Ce2O3 1.07, Pr2O3 0.20, Nd2O3 0.44, Al2O3 0.29, SiO2 50.81, ZrO2 13.50, HfO2 0.45, TiO2 0.61, Nb2О5 1.10, Cl 1.01, SO3 0.29, H2O 8.10, –O≡Cl –0.23, total 99.68. The empirical formula is H25.94Na6.03K0.16Mg0.07Ca3.51Sr0.52Ce0.19La0.10Nd0.08Pr0.03Mn1.91Fe0.47Ti0.22Zr3.16Hf0.06Nb0.24Si24.40Al0.16S0.10Cl0.82O79.13. The crystal structure was determined using single-crystal X-ray diffraction data and refined to R = 0.0484. Selsurtite is trigonal, space group R3, with a = 14.1475(7) Å, c = 30.3609(12) Å, V = 5262.65(7) Å3 and Z = 3. Infrared and Raman spectra show that hydronium cations are involved in very strong hydrogen bonds and form Zundel- and Eigen-like complexes. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %)(hkl)] are: 11.38 (56)(101), 7.08 (59)(110), 5.69 (36)(202), 4.318 (72)(205), 3.793 (36)(303), 3.544 (72)(027, 220, 009), 2.970 (100)(315) and 2.844 (100)(404). The mineral is named after the discovery locality.
1}. Selsurtite is brittle, with the Mohs’ hardness of 5. No cleavage is observed. Parting is distinct on (001). D(meas) = 2.73(2) and D(calc) = 2.722 g⋅cm–3. Selsurtite is optically uniaxial (–), with ω = 1.598(2) and ɛ = 1.595(2). The chemical composition is (wt.%, electron microprobe): Na2O 6.48, K2O 0.27, MgO 0.10, CaO 6.83, MnO 4.73, FeO 1.18, SrO 1.88, La2O3 0.57, Ce2O3 1.07, Pr2O3 0.20, Nd2O3 0.44, Al2O3 0.29, SiO2 50.81, ZrO2 13.50, HfO2 0.45, TiO2 0.61, Nb2О5 1.10, Cl 1.01, SO3 0.29, H2O 8.10, –O≡Cl –0.23, total 99.68. The empirical formula is H25.94Na6.03K0.16Mg0.07Ca3.51Sr0.52Ce0.19La0.10Nd0.08Pr0.03Mn1.91Fe0.47Ti0.22Zr3.16Hf0.06Nb0.24Si24.40Al0.16S0.10Cl0.82O79.13. The crystal structure was determined using single-crystal X-ray diffraction data and refined to R = 0.0484. Selsurtite is trigonal, space group R3, with a = 14.1475(7) Å, c = 30.3609(12) Å, V = 5262.65(7) Å3 and Z = 3. Infrared and Raman spectra show that hydronium cations are involved in very strong hydrogen bonds and form Zundel- and Eigen-like complexes. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %)(hkl)] are: 11.38 (56)(101), 7.08 (59)(110), 5.69 (36)(202), 4.318 (72)(205), 3.793 (36)(303), 3.544 (72)(027, 220, 009), 2.970 (100)(315) and 2.844 (100)(404). The mineral is named after the discovery locality.
Associate Editor: Anthony R Kampf