Published online by Cambridge University Press: 17 March 2020
The new mineral pseudomeisserite-(NH4) (IMA2018-166), (NH4,K)2Na4[(UO2)2(SO4)5]⋅4H2O, was found in the Blue Lizard mine, San Juan County, Utah, USA, where it occurs as light yellow prisms in a secondary assemblage with belakovskiite, blödite, changoite, ferrinatrite, gypsum, ivsite, metavoltine and tamarugite. The streak is very pale yellow and the fluorescence is bright lime green under 405 nm ultraviolet light. Crystals are transparent with vitreous lustre. The tenacity is brittle, the Mohs hardness is 2½, the fracture is curved or conchoidal and there is one perfect cleavage on {100}. The mineral is easily soluble in H2O and has a measured density of 3.22(2) g⋅cm–3. Pseudomeisserite-(NH4) is optically biaxial (–) with α = 1.536(2), β = 1.559(2) and γ = 1.565(2) (white light); 2Vmeas. = 53(1)°; dispersion is r > v, distinct; pleochroism: X colourless, Y light yellow and Z pale yellow (X < Z < Y); optical orientation: Z = b, Y ∧ c = 33° in obtuse β). Electron microprobe analyses (WDS mode) provided (NH4)1.49K0.60Na3.87U2.00S5.04O28H7.78. The five strongest X-ray powder diffraction lines are [dobs, Å(I)(hkl)]: 12.69(76)(100), 6.83(84)(012,102), 6.01(100)(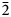 $\bar{2}$02), 3.959(67)(
$\bar{2}$02), 3.959(67)(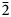 $\bar{2}$21,
$\bar{2}$21,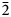 $\bar{2}$14,
$\bar{2}$14,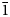 $\bar{1}$23) and 3.135(76)(
$\bar{1}$23) and 3.135(76)(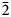 $\bar{2}$06,223,
$\bar{2}$06,223,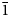 $\bar{1}$16). Pseudomeisserite-(NH4) is monoclinic, P21/c, a = 13.1010(3), b = 10.0948(2), c = 19.4945(14) Å, β = 104.285(7)°, V = 2498.5(2) Å3 and Z = 4. The structural unit in the structure (R1 = 0.0254 for 3837 I > 2σI reflections) is a novel [(UO2)2(SO4)5]6– uranyl-sulfate band.
$\bar{1}$16). Pseudomeisserite-(NH4) is monoclinic, P21/c, a = 13.1010(3), b = 10.0948(2), c = 19.4945(14) Å, β = 104.285(7)°, V = 2498.5(2) Å3 and Z = 4. The structural unit in the structure (R1 = 0.0254 for 3837 I > 2σI reflections) is a novel [(UO2)2(SO4)5]6– uranyl-sulfate band.
Associate Editor: Daniel Atencio