Article contents
A new mineral, scotlandite (PbSO3) from Leadhills, Scotland; the first naturally occurring sulphite
Published online by Cambridge University Press: 05 July 2018
Abstract
Electron microprobe analysis of the new mineral scotlandite yielded Pb 72.27, S 9.95, corresponding to PbO 77.85, SO2 19.88, sum 97.73 wt.%. Secondary ion mass spectrometry showed the absence of Li, Be, B, C, N, and F, but the presence of very small amounts of Br. Infrared spectroscopy showed that the mineral is a sulphite with neither OH nor any other polyatomic anions. The empirical formula, calculated on the basis of Pb+S = 2 is Pb1.06S0.94O2.94 or ideally PbSO3.
The mineral has the following vibrations of the sulphite ion in the infrared spectrum: v3 920, 865; v1 970 (?); v2 620, 600; v4 488, 470 cm−1.
Scotlandite is monoclinic, with possible space groups P21 or P21/m. The unit cell dimensions are: a 4.542(2), b 5.333(2), c 6.413(2) Å, β 106.22(4)°, Z = 2. The strongest lines in the powder diffraction pattern are: 3.99(10) (011), 3.38(7) (110), 3.25(8) (11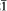 ), 3.07(4) (002), 2.66(7) (020, 012), 2.56(4) (11
), 3.07(4) (002), 2.66(7) (020, 012), 2.56(4) (11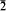 ), 2.24(5) (12
), 2.24(5) (12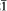 , 102), 2.01(5)(210, 022), 1.707(4) (031), 1.538(4) (032, 004).
, 102), 2.01(5)(210, 022), 1.707(4) (031), 1.538(4) (032, 004).
Scotlandite occurs as chisel-shaped or bladed crystals elongated along the c-axis, with a tendency to form radiating clusters. The following forms have been determined: {100}, {010}, {011}, {021}, {031}, and {032}. The new mineral has a pronounced cleavage along {100}, and a less good one along {010}. The crystals are pale yellow to greyish-white and colourless, sometimes transparent. Their lustre is adamantine, pearly on cleavage planes.
The mineral is optically biaxial positive, 2Vmeas. 35° 24' (Na). The refractive indices are: α ~ 2.035, β ~ 2.040, and γ ~ 2.085 (Na). Dispersion is strong, v >> r. The extinction is β//b, and α [001] = 20° (γ: [100] = 4°) in the obtuse angle β. H (Mohs) ≤ 2. D = 6.37 and calculated Dx = 6.40 g cm-3.
Scotlandite occurs in cavities in massive baryte and anglesite, and is closely associated with lanarkite and susannite; it represents the latest phase in the crystallization sequence of the associated lead secondary minerals. The label locality of the specimen is the Susanna vein, Leadhills, Scotland.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1984
References
- 22
- Cited by


