Published online by Cambridge University Press: 05 July 2018
Nestolaite (IMA 2013-074), CaSeO3·H2O, is a new mineral species from the Little Eva mine, Grand County, Utah, USA. It is named in honour of the prominent Italian mineralogist and crystallographer Fabrizio Nestola. The new mineral was found on sandstone matrix as rounded aggregates up to 2 mm across and up to 0.05 μm thick consisting of tightly intergrown oblique-angled, flattened to acicular crystals up to 30 μm long and up to 7 μm (very rarely up to 15 μm) thick. Nestolaite associates with cobaltomenite, gypsum, metarossite, orschallite and rossite. The new mineral is light violet and transparent with a white streak and vitreous lustre. The Mohs hardness is 2½. Nestolaite is brittle, has uneven fracture and perfect cleavage on {100}. The measured and calculated densities are Dmeas. = 3.18(2) g/cm3 and Dcalc. = 3.163 g/cm3. Optically, nestolaite is biaxial positive. The refractive indices are α = 1.642(3), β = 1.656(3), γ = 1.722(6). The measured 2V is 55(5)° and the calculated 2V is 51°. In transmitted light nestolaite is colourless. It does not show pleochroism but has strong pseudoabsorption caused by high birefringence. The chemical composition of nestolaite (wt.%, electronmicroprobe data) is: CaO 28.97, SeO2 61.14, H2O (calc.) 9.75, total 99.86. The empirical formula calculated on the basis of 4 O a.p.f.u. (atoms per formula unit) is Ca0.96Se1.02O3·H2O. The Raman spectrum is dominated by the Se–O stretching and O–Se–O bending vibrations of the pyramidal SeO3 groups and O–H stretching modes of the H2O molecules. The mineral is monoclinic, space group P21/c, with a = 7.6502(9), b = 6.7473(10), c = 7.9358(13) Å, β = 108.542 (12)°, V = 388.37(10) Å3 and Z = 4. The eight strongest powder X-ray diffraction lines are [dobs in Å(hkl) (Irel)]: 7.277 (100)(100), 4.949 (110)(37), 3.767 (002)(29), 3.630 (200)(58), 3.371 (020)(24), 3.163 ( 02)(74), 2.9783 (
02)(74), 2.9783 (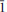 21)(74) and 2.7231 (112)(31). The crystal structure of nestolaite was determined by means of the Rietveld refinement from the powder data to Rwp = 0.019. Nestolaite possesses a layered structure consisting of CaΦ–SeO3 sheets, composed of edge-sharing polyhedra. Adjacent sheets are held by H bonds emanating from the single (H2O) group within the sheets. The nestolaite structure is topologically unique.
21)(74) and 2.7231 (112)(31). The crystal structure of nestolaite was determined by means of the Rietveld refinement from the powder data to Rwp = 0.019. Nestolaite possesses a layered structure consisting of CaΦ–SeO3 sheets, composed of edge-sharing polyhedra. Adjacent sheets are held by H bonds emanating from the single (H2O) group within the sheets. The nestolaite structure is topologically unique.