Published online by Cambridge University Press: 17 March 2021
A common attribute of two rare natural chromium-bearing oxides: mcconnellite, CuCrO2, discovered more than 40 years ago in Guyana, and a new mineral ellinaite, CaCr2O4, described from two localities (Brazil and Israel) recently, is that these minerals are poorly studied due to their rarity and small size. Mcconnellite and ellinaite in the present study were found in varicoloured marbles of the pyrometamorphic Hatrurim Complex in the Tulul Al Hammam area, Daba-Siwaqa, Jordan. Structural data obtained for ellinaite (Pnma, a = 9.0875(2), b = 2.9698(1), c = 10.6270(3) Å and V = 286.80(2) Å3), with an empirical formula (Сa1.00Sr0.01)Σ1.01(Cr3+1.79Al0.07Ca0.04Fe3+0.04 Ti4+0.03Mg0.03)Σ2.00O4, is similar to the structural data of synthetic analogue β-CaCr2O4 but differs significantly from the data obtained for ellinaite from Brazil and Israel despite the fact that all the natural phases have a similar composition. Single-crystal X-ray diffraction data for mcconnellite from Jordan (R$\bar{3}$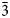 m, a = b = 2.9756(1) and c = 17.124(1) Å) with composition (Cu0.98Ca0.03Sr0.01)Σ1.02(Cr3+0.90Al0.05 Fe3+0.03)Σ0.98O2, is the first determination of a natural Cu-delafossite-type structure. This paper also presents the results of a single-crystal Raman study for mcconnellite and ellinaite, which indicates that the spectral features of these minerals is dependent on crystal orientation.
m, a = b = 2.9756(1) and c = 17.124(1) Å) with composition (Cu0.98Ca0.03Sr0.01)Σ1.02(Cr3+0.90Al0.05 Fe3+0.03)Σ0.98O2, is the first determination of a natural Cu-delafossite-type structure. This paper also presents the results of a single-crystal Raman study for mcconnellite and ellinaite, which indicates that the spectral features of these minerals is dependent on crystal orientation.
Associate Editor: G. Diego Gatta