Published online by Cambridge University Press: 05 July 2018
MacFallite, Ca2(Mn3+,Al)3(OH)3[SiO4] [Si2O7], is a new species occurring with manganite, braunite, orientite and pyrolusite blebs, stringers, and framboidal aggregates, which replace calcite in fissures and lenses in Keweenaw basalt near Manganese Lake, Copper Harbor, Keweenaw County, Michigan. It is rich reddish brown to maroon in coarse aggregates; compact massive material is brown to dull pink. The streak and powder are brown with a reddish tint. Lustre silky to subadamantine, specific gravity 3.43(2), hardness 5+, cleavage {001} perfect; twinning by reflection on {100} is universal. The mineral is monoclinic, space group P21, or P21/m, Z = 2, a 8.929(6), b 6.045(5), c 10.905(7) Å, β 119.10(3)°, α 1.773(5), β 1.795(5), γ 1.815(5), sign + or −, pleochroism α yellow, β light brown, γ dark brown, γ‖b. Orientite, Ca2Mn 2+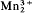 (OH)4[Si3O10]-Ca2
(OH)4[Si3O10]-Ca2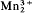 (OH)2[Si3O10] ·2H2O, orthorhombic, space group Cc2m, Ccm21or Ccmm, a 9.042(4), b 6.090(2), c 18.990(7) Å, α 1.765(5), β 1.79(1), γ 1.81(1), sign + or −, α brownish yellow, β reddish brown, γ deep brownish red, α‖a, γ‖b, also occurs in moderate abundance. It is turbid chocolate brown in platy masses but crystals are transparent rich reddish brown. Streak and powder brown. Observed forms are m{110}, c{001}, and poorly developed to absent α{100}, thin to thick tabular parallel to {001}, cleavage (or parting) {001} perfect, specific gravity 3.33.
(OH)2[Si3O10] ·2H2O, orthorhombic, space group Cc2m, Ccm21or Ccmm, a 9.042(4), b 6.090(2), c 18.990(7) Å, α 1.765(5), β 1.79(1), γ 1.81(1), sign + or −, α brownish yellow, β reddish brown, γ deep brownish red, α‖a, γ‖b, also occurs in moderate abundance. It is turbid chocolate brown in platy masses but crystals are transparent rich reddish brown. Streak and powder brown. Observed forms are m{110}, c{001}, and poorly developed to absent α{100}, thin to thick tabular parallel to {001}, cleavage (or parting) {001} perfect, specific gravity 3.33.
A fumarolic origin is proposed for the assemblage, which in many respects is similar to the great manganese oxide deposits in Oriente Province, Cuba. Macfallite appears to be structurally related to pumpellyite while orientite is apparently related to ardennite.
Died 6 June 1978.