Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Rosner, H.
Johannes, M.D.
Drechsler, S.-L
Schmitt, M.
Janson, O.
Schnelle, W.
Liu, W.
Huang, Y.-X
and
Kniep, R.
2007.
CuII materials—From crystal chemistry to magnetic model compounds.
Science and Technology of Advanced Materials,
Vol. 8,
Issue. 5,
p.
352.
Colman, R. H.
Ritter, C.
and
Wills, A. S.
2008.
Toward Perfection: Kapellasite, Cu3Zn(OH)6Cl2, a New Model S = 1/2 Kagome Antiferromagnet.
Chemistry of Materials,
Vol. 20,
Issue. 22,
p.
6897.
Janson, O.
Richter, J.
and
Rosner, H.
2008.
Modified Kagome Physics in the Natural Spin-1/2Kagome Lattice Systems: KapellasiteCu3Zn(OH)6Cl2and HaydeeiteCu3Mg(OH)6Cl2.
Physical Review Letters,
Vol. 101,
Issue. 10,
Janson, O
Richter, J
and
Rosner, H
2009.
Intrinsic peculiarities of real material realizations of a spin-1/2 kagomé lattice.
Journal of Physics: Conference Series,
Vol. 145,
Issue. ,
p.
012008.
Greedan, John E.
2010.
Functional Oxides.
p.
41.
Colman, R.H.
Sinclair, A.
and
Wills, A.S.
2010.
Comparisons between Haydeeite, α-Cu3Mg(OD)6Cl2, and Kapellasite, α-Cu3Zn(OD)6Cl2, Isostructural S = 1/2 Kagome Magnets.
Chemistry of Materials,
Vol. 22,
Issue. 20,
p.
5774.
Chu, Shaoyan
Müller, Peter
Nocera, Daniel G.
and
Lee, Young S.
2011.
Hydrothermal growth of single crystals of the quantum magnets: Clinoatacamite, paratacamite, and herbertsmithite.
Applied Physics Letters,
Vol. 98,
Issue. 9,
Colman, R. H.
Sinclair, A.
and
Wills, A. S.
2011.
Magnetic and Crystallographic Studies of Mg-Herbertsmithite, γ-Cu3Mg(OH)6Cl2—A New S = 1/2 Kagome Magnet and Candidate Spin Liquid.
Chemistry of Materials,
Vol. 23,
Issue. 7,
p.
1811.
Mendels, Philippe
and
Wills, Andrew S.
2011.
Introduction to Frustrated Magnetism.
Vol. 164,
Issue. ,
p.
207.
Chu, Shaoyan
2011.
Magnetic properties of geometrically frustrated polymorphic crystals of Cu4-xMgx(OH)6Cl2.
Journal of Physics: Conference Series,
Vol. 273,
Issue. ,
p.
012123.
McQueen, T.M.
Han, T.H.
Freedman, D.E.
Stephens, P.W.
Lee, Y.S.
and
Nocera, D.G.
2011.
CdCu3(OH)6Cl2: A new layered hydroxide chloride.
Journal of Solid State Chemistry,
Vol. 184,
Issue. 12,
p.
3319.
Jeschke, Harald O.
Salvat-Pujol, Francesc
and
Valentí, Roser
2013.
First-principles determination of Heisenberg Hamiltonian parameters for the spin-12kagome antiferromagnet ZnCu3(OH)6Cl2.
Physical Review B,
Vol. 88,
Issue. 7,
Kampf, A. R.
Sciberras, M. J.
Leverett, P.
Williams, P. A.
Malcherek, T.
Schlüter, J.
Welch, M. D.
Dini, M.
and
Molina Donoso, A. A.
2013.
Paratacamite-(Mg), Cu3(Mg,Cu)Cl2(OH)6; a new substituted basic copper chloride mineral from Camerones, Chile.
Mineralogical Magazine,
Vol. 77,
Issue. 8,
p.
3113.
Majoni, Stephen
and
Hossenlopp, Jeanne M.
2014.
Controlled Release Kinetics in Hydroxy Double Salts: Effect of Host Anion Structure.
Advances in Physical Chemistry,
Vol. 2014,
Issue. ,
p.
1.
Malcherek, T.
Bindi, L.
Dini, M.
Ghiara, M. R.
Molina Donoso, A.
Nestola, F.
Rossi, M.
and
Schlüter, J.
2014.
Tondiite, Cu3Mg(OH)6Cl2, the Mg-analogue of herbertsmithite.
Mineralogical Magazine,
Vol. 78,
Issue. 3,
p.
583.
Welch, Mark D.
Sciberras, Matthew J.
Williams, Peter A.
Leverett, Peter
Schlüter, Jochen
and
Malcherek, Thomas
2014.
A temperature-induced reversible transformation between paratacamite and herbertsmithite.
Physics and Chemistry of Minerals,
Vol. 41,
Issue. 1,
p.
33.
Iqbal, Yasir
Jeschke, Harald O.
Reuther, Johannes
Valentí, Roser
Mazin, I. I.
Greiter, Martin
and
Thomale, Ronny
2015.
Paramagnetism in the kagome compounds(Zn,Mg,Cd)Cu3(OH)6Cl2.
Physical Review B,
Vol. 92,
Issue. 22,
Sun, Wei
Huang, Ya-Xi
Pan, Yuanming
and
Mi, Jin-Xiao
2016.
Synthesis and magnetic properties of centennialite: a new S = ½ Kagomé antiferromagnet and comparison with herbertsmithite and kapellasite.
Physics and Chemistry of Minerals,
Vol. 43,
Issue. 2,
p.
127.
Crichton, Wilson A.
and
Müller, Harald
2017.
Centennialite, CaCu3(OH)6Cl2.nH2O,n≈ 0.7, a new kapellasite-like species, and a reassessment of calumetite.
Mineralogical Magazine,
Vol. 81,
Issue. 5,
p.
1105.
Okuma, Ryutaro
Yajima, Takeshi
Nishio-Hamane, Daisuke
Okubo, Tsuyoshi
and
Hiroi, Zenji
2017.
Weak ferromagnetic order breaking the threefold rotational symmetry of the underlying kagome lattice in
CdCu3(OH)6(NO3)2·H2O.
Physical Review B,
Vol. 95,
Issue. 9,
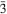 ml, unit-cell parameters (from single-crystal data) a = 6.300(1), c = 5.733(1) Å, V= 197.06(6) Å3, Z = 1. The crystal structure of kapellasite is based on brucite-like sheets parallel to (0001), built from edge-sharing distorted M(OH,Cl)6 (M = Cu, Zn) octahedra. The sheets stack directly on each other (…AAA… stacking). Bonding between adjacent sheets is only due to weak hydrogen and O…C1 bonds. The name is in honour of Christo Kapellas (1938–2004), collector and mineral dealer from Kamariza, Lavrion, Greece.
ml, unit-cell parameters (from single-crystal data) a = 6.300(1), c = 5.733(1) Å, V= 197.06(6) Å3, Z = 1. The crystal structure of kapellasite is based on brucite-like sheets parallel to (0001), built from edge-sharing distorted M(OH,Cl)6 (M = Cu, Zn) octahedra. The sheets stack directly on each other (…AAA… stacking). Bonding between adjacent sheets is only due to weak hydrogen and O…C1 bonds. The name is in honour of Christo Kapellas (1938–2004), collector and mineral dealer from Kamariza, Lavrion, Greece.