Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kamath, P.Vishnu
Annal Therese, G.Helen
and
Gopalakrishnan, J.
1997.
On the Existence of Hydrotalcite-Like Phases in the Absence of Trivalent Cations.
Journal of Solid State Chemistry,
Vol. 128,
Issue. 1,
p.
38.
Refait, PH.
Abdelmoula, M.
and
GÉnin, J.-M.R.
1998.
Mechanisms of formation and structure of green rust one in aqueous corrosion of iron in the presence of chloride ions.
Corrosion Science,
Vol. 40,
Issue. 9,
p.
1547.
Grguric, B. A.
Madsen, I. C.
and
Pring, A.
2001.
Woodallite, a new chromium analogue of iowaite from the Mount Keith nickel deposit, Western Australia.
Mineralogical Magazine,
Vol. 65,
Issue. 3,
p.
427.
Génin, Jean-Marie R.
Refait, Philippe
Bourrié, Guilhem
Abdelmoula, Mustapha
and
Trolard, Fabienne
2001.
Structure and stability of the Fe(II)–Fe(III) green rust “fougerite” mineral and its potential for reducing pollutants in soil solutions.
Applied Geochemistry,
Vol. 16,
Issue. 5,
p.
559.
Simon, Lilian
François, Michel
Refait, Philippe
Renaudin, Guillaume
Lelaurain, Michèle
and
Génin, Jean-Marie R
2003.
Structure of the Fe(II-III) layered double hydroxysulphate green rust two from Rietveld analysis.
Solid State Sciences,
Vol. 5,
Issue. 2,
p.
327.
Evans, David G.
and
Slade, Robert C. T.
2005.
Layered Double Hydroxides.
Vol. 119,
Issue. ,
p.
1.
Frost, Ray L.
Adebajo, Moses O.
and
Erickson, Kristy L.
2005.
Raman spectroscopy of synthetic and natural iowaite.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 61,
Issue. 4,
p.
613.
Génin, Jean-Marie R.
Aïssa, Rabha
Géhin, Antoine
Abdelmoula, Mustapha
Benali, Omar
Ernstsen, Vibeke
Ona-Nguema, Georges
Upadhyay, Chandan
and
Ruby, Christian
2005.
Fougerite and FeII–III hydroxycarbonate green rust; ordering, deprotonation and/or cation substitution; structure of hydrotalcite-like compounds and mythic ferrosic hydroxide.
Solid State Sciences,
Vol. 7,
Issue. 5,
p.
545.
Bouzaid, J. M.
Frost, R. L.
Musumeci, A. W.
and
Martens, W. N.
2006.
Thermal decomposition of the synthetic hydrotalcite woodallite.
Journal of Thermal Analysis and Calorimetry,
Vol. 86,
Issue. 3,
p.
745.
Trolard, Fabienne
Bourrié, Guilhem
Abdelmoula, Mustapha
Refait, Philippe
and
Feder, Frédéric
2007.
Fougerite, a new mineral of the pyroaurite-iowaite group: Description and crystal structure.
Clays and Clay Minerals,
Vol. 55,
Issue. 3,
p.
323.
Villars, P.
Cenzual, K.
Daams, J.
Gladyshevskii, R.
Shcherban, O.
Dubenskyy, V.
Melnichenko-Koblyuk, N.
Pavlyuk, O.
Savysyuk, I.
Stoyko, S.
and
Sysa, L.
2007.
Structure Types. Part 5: Space Groups (173) P63 - (166) R-3m.
Vol. 43A5,
Issue. ,
p.
606.
Chukanov, N. V.
Pekov, I. V.
Levitskaya, L. A.
and
Zadov, A. E.
2009.
Droninoite, Ni3Fe3+Cl(OH)8 · 2H2O, a new hydrotalcite-group mineral species from the weathered Dronino meteorite.
Geology of Ore Deposits,
Vol. 51,
Issue. 8,
p.
767.
Lakshmi Reddy, S.
Sesha Maheswaramma, K.
Siva Reddy, G.
Reddy, B. J.
Endo, Tamio
and
Frost, Ray L.
2010.
Optical absorption, infrared, Raman, and EPR spectral studies on natural iowaite mineral.
Transition Metal Chemistry,
Vol. 35,
Issue. 3,
p.
331.
Mills, S. J.
Wilson, S. A.
Dipple, G. M.
and
Raudsepp, M.
2010.
The decomposition of konyaite: importance in CO2fixation in mine tailings.
Mineralogical Magazine,
Vol. 74,
Issue. 5,
p.
903.
Krivovichev, S. V.
Yakovenchuk, V. N.
Zhitova, E. S.
Zolotarev, A. A.
Pakhomovsky, Y. A.
and
Ivanyuk, G. Yu.
2010.
Crystal chemistry of natural layered double hydroxides. I. Quintinite-2H-3c from the Kovdor alkaline massif, Kola peninsula, Russia.
Mineralogical Magazine,
Vol. 74,
Issue. 5,
p.
821.
Krivovichev, Sergey V.
Yakovenchuk, Victor N.
and
Zhitova, Elena S.
2011.
Minerals as Advanced Materials II.
p.
87.
Manohara, G. V.
Prasanna, S. V.
and
Kamath, P. Vishnu
2011.
Structure and Composition of the Layered Double Hydroxides of Mg and Fe: Implications for Anion‐Exchange Reactions.
European Journal of Inorganic Chemistry,
Vol. 2011,
Issue. 16,
p.
2624.
Mills, S. J.
Christy, A. G.
Génin, J.-M. R.
Kameda, T.
and
Colombo, F.
2012.
Nomenclature of the hydrotalcite supergroup: natural layered double hydroxides.
Mineralogical Magazine,
Vol. 76,
Issue. 5,
p.
1289.
Richardson, Ian G.
2013.
The importance of proper crystal-chemical and geometrical reasoning demonstrated using layered single and double hydroxides.
Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials,
Vol. 69,
Issue. 2,
p.
150.
Gole, Martin J.
2014.
Leaching of S, Cu, and Fe from disseminated Ni-(Fe)-(Cu) sulphide ore during serpentinization of dunite host rocks at Mount Keith, Agnew-Wiluna belt, Western Australia.
Mineralium Deposita,
Vol. 49,
Issue. 7,
p.
821.
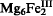 (OH)16Cl2·4H2O. It is trigonal, R3̄m, a = 3.1183(9), c = 24.113(8) Å, V = 203.1(2) Å3, Z = 3/8; Dobs 2.09 g/cm3.; Dcalc 2.04 g/cm3; hardness (Mohs)=2 1/2. The interlayer Cl− ions are displaced from the threefold axis. It is uniaxial negative, with ω = 1.561 ± 0.002, ε = 1.543 ± 0.002; coloured crystals are intensely pleochroic, due to intervalence charge transfer between the Fe3+ and Fe2+ substituting for Mg2+, with O pale yellow, E deep blue-green.
(OH)16Cl2·4H2O. It is trigonal, R3̄m, a = 3.1183(9), c = 24.113(8) Å, V = 203.1(2) Å3, Z = 3/8; Dobs 2.09 g/cm3.; Dcalc 2.04 g/cm3; hardness (Mohs)=2 1/2. The interlayer Cl− ions are displaced from the threefold axis. It is uniaxial negative, with ω = 1.561 ± 0.002, ε = 1.543 ± 0.002; coloured crystals are intensely pleochroic, due to intervalence charge transfer between the Fe3+ and Fe2+ substituting for Mg2+, with O pale yellow, E deep blue-green.