No CrossRef data available.
Published online by Cambridge University Press: 05 July 2018
Heyite occurs in silicified limestone with other oxidation-zone minerals derived from galena, chalcopyrite, and pyrite. The occurrence is near Ely, Nevada. Most common are pyromorphite, cerussite, and chrysocolla. Heyite occurs on and replaces corroded tungstenian wulfenite. Crystals are small (up to 0·4 mm), simple in habit, and monoclinic. Only {001}, {100}, {110}, and {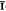 01} have been observed. Twinning on {110} is relatively common. The colour is yellow-orange with a yellow streak, H = 4. Electron probe analyses (average of three) gave PbO 75·4 %, ZnO 0·81 %, FeO 8·41 %, V2O5 12·65 %. This leads to Pb5(Fe,Zn)2(VO4)2O4, with Z = 1.
01} have been observed. Twinning on {110} is relatively common. The colour is yellow-orange with a yellow streak, H = 4. Electron probe analyses (average of three) gave PbO 75·4 %, ZnO 0·81 %, FeO 8·41 %, V2O5 12·65 %. This leads to Pb5(Fe,Zn)2(VO4)2O4, with Z = 1.
The space group is P21/m. Cell constants, refined from powder data, are a 8·910 Å, b 6·017, c 7·734 (all ±0·004 Å); β III° 53′±4′. The strongest powder lines (Cr-Kα) are: 3·248, 11 , (100); 2·970, 30
, (100); 2·970, 30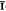 , 21
, 21 (69); 2·767, 211 (61); 4·873,110, (46); 3·674, 111, (35); 2·306 (33); 3·010, 020, (29); 3·412, 20
(69); 2·767, 211 (61); 4·873,110, (46); 3·674, 111, (35); 2·306 (33); 3·010, 020, (29); 3·412, 20 , 11
, 11 , (25); 8·281, 100, (25); and 2·113 (20). Gcalc 6·284, Gmeas 6·3±0·2 Optically biaxial with 2Vγ 82°calc, 89°meas; αD 2·185, βD 2·219, γD 2·266; all values ±0·01 and determined in S-Se melts. Dispersion is weak with π > ν. The orientation is β = b, α: [001] 36° (in obtuse β). Nonpleochroic.
, (25); 8·281, 100, (25); and 2·113 (20). Gcalc 6·284, Gmeas 6·3±0·2 Optically biaxial with 2Vγ 82°calc, 89°meas; αD 2·185, βD 2·219, γD 2·266; all values ±0·01 and determined in S-Se melts. Dispersion is weak with π > ν. The orientation is β = b, α: [001] 36° (in obtuse β). Nonpleochroic.
Three small specimens were found, and represent a total of less than 100 mg of the mineral. One has been deposited with the British Museum (Natural History). The name is for Dr. Max Hey, eminent British chemist and mineralogist.
The cell is strikingly similar to that of brackebuschite but electron probe examination of a newly analysed (by atomic absorption) specimen of the latter from the type locality in Argentina shows a marked chemical difference. Heyite strongly resembles descloizite in appearance.