Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Smith, Douglas
and
Boyd, Francis R.
1992.
Compositional zonation in garnets in peridotite xenoliths.
Contributions to Mineralogy and Petrology,
Vol. 112,
Issue. 1,
p.
134.
Snyder, Gregory A.
Taylor, Lawrence A.
Jerde, Eric A.
Sharkov, Yevgeniy
Laz'ko, Yevgeniy
and
Hanna, S.
1993.
Petrogenesis of Garnet Pyroxenite and Spinel Peridotite Xenoliths of the Tell-Danun Alkali Basalt Volcano, Harrat As Shamah, Syria.
International Geology Review,
Vol. 35,
Issue. 12,
p.
1104.
Canil, Dante
1994.
An experimental calibration of the ?Nickel in Garnet? geothermometer with applications.
Contributions to Mineralogy and Petrology,
Vol. 117,
Issue. 4,
p.
410.
Sutherland, F. L.
Raynor, L. R.
and
Pogson, R. E.
1994.
Spinel to garnet lherzolite transition in relation to high temperature palaeogeotherms, eastern Australia∗.
Australian Journal of Earth Sciences,
Vol. 41,
Issue. 3,
p.
205.
Sharkov, E. V.
Bogina, M. M.
Quick, J. E.
and
Mekhonoshin, A. S.
1995.
Tectonic Blocks of the Precambrian Lower Crust and Upper Mantle, Southern Sayan Mountains, East Siberia.
International Geology Review,
Vol. 37,
Issue. 1,
p.
81.
Hiramatsu, N.
Banno, S.
Hirajima, T.
and
Cong, B.
1995.
Ultrahigh‐pressure garnet lherzolite from Chijiadian, Rongcheng County, in the Su‐Lu region of eastern China.
Island Arc,
Vol. 4,
Issue. 4,
p.
324.
Van Roermund
and
Drury
1998.
Ultra‐high pressure (P > 6 GPa) garnet peridotites in Western Norway: exhumation of mantle rocks from > 185 km depth.
Terra Nova,
Vol. 10,
Issue. 6,
p.
295.
Kopylova, M. G.
Russell, J. K.
and
Cookenboo, H.
1999.
Petrology of Peridotite and Pyroxenite Xenoliths from the Jericho Kimberlite: Implications for the Thermal State of the Mantle beneath the Slave Craton, Northern Canada.
Journal of Petrology,
Vol. 40,
Issue. 1,
p.
79.
Gasparik, Tibor
2000.
An Internally Consistent Thermodynamic Model for the System CaO‐MgO‐Al2O3‐SiO2 Derived Primarily from Phase Equilibrium Data.
The Journal of Geology,
Vol. 108,
Issue. 1,
p.
103.
Simakov, Sergei K.
and
Taylor, Lawrence A.
2000.
Geobarometry for Mantle Eclogites: Solubility Of Ca-Tschermaks in Clinopyroxene.
International Geology Review,
Vol. 42,
Issue. 6,
p.
534.
Pearson, D.G.
Canil, D.
and
Shirey, S.B.
2003.
Treatise on Geochemistry.
p.
171.
Enami, M.
Mizukami, T.
and
Yokoyama, K.
2004.
Metamorphic evolution of garnet‐bearing ultramafic rocks from the Gongen area, Sanbagawa belt, Japan.
Journal of Metamorphic Geology,
Vol. 22,
Issue. 1,
p.
1.
James, D. E.
Boyd, F. R.
Schutt, D.
Bell, D. R.
and
Carlson, R. W.
2004.
Xenolith constraints on seismic velocities in the upper mantle beneath southern Africa.
Geochemistry, Geophysics, Geosystems,
Vol. 5,
Issue. 1,
Carswell, D. A.
van Roermund, H. L.M.
and
de Vries, D. F.Wiggers
2006.
Scandian Ultrahigh-Pressure Metamorphism of Proterozoic Basement Rocks on Fjørtoft and Otrøy, Western Gneiss Region, Norway.
International Geology Review,
Vol. 48,
Issue. 11,
p.
957.
Michaut, C.
Jaupart, C.
and
Bell, D. R.
2007.
Transient geotherms in Archean continental lithosphere: New constraints on thickness and heat production of the subcontinental lithospheric mantle.
Journal of Geophysical Research: Solid Earth,
Vol. 112,
Issue. B4,
Glebovitskii, V. A.
Nikitina, L. P.
Saltykova, A. K.
Pushkarev, Yu. D.
Ovchinnikov, N. O.
Babushkina, M. S.
and
Ashchepkov, I. V.
2007.
Thermal and chemical heterogeneity of the upper mantle beneath the Baikal-Mongolia territory.
Petrology,
Vol. 15,
Issue. 1,
p.
58.
Etzel, Katja
Benisek, Artur
Dachs, Edgar
and
Cemič, Lado
2007.
Thermodynamic mixing behavior of synthetic Ca-Tschermak–diopside pyroxene solid solutions: I. Volume and heat capacity of mixing.
Physics and Chemistry of Minerals,
Vol. 34,
Issue. 10,
p.
733.
Perchuk, L. L.
Gerya, T. V.
Parfenova, O. V.
and
Podgornova, S. T.
2007.
Metamorphic rocks of the Samerberg complex, Eastern Alps: 2. P-T paths and the problem of a geodynamic model.
Petrology,
Vol. 15,
Issue. 4,
p.
369.
POWELL, R.
and
HOLLAND, T. J. B.
2008.
On thermobarometry.
Journal of Metamorphic Geology,
Vol. 26,
Issue. 2,
p.
155.
Roermund, Herman van
2009.
Recent progress in Scandian ultrahigh-pressure metamorphism in the northernmost domain of the Western Gneiss Complex, SW Norway: continental subduction down to 180–200 km depth.
Journal of the Geological Society,
Vol. 166,
Issue. 4,
p.
739.
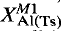 orthopyroxene contents are presented. Application to analytical data sets for garnet lherzolite zenolith suites in the Thaba Putsoa and Mothae kimberlites generates revised upper mantle P-T arrays which refute the widely accepted advocacy by Finnerty and Boyd (1984, 1987) and Finnerty (1989) of an upper-mantle palaeogeotherm beneath northern Lesotho that is markedly inflected to a higher thermal gradient at the depths of derivation of the more chemically fertile, porphyroclastic textured, xenoliths.
orthopyroxene contents are presented. Application to analytical data sets for garnet lherzolite zenolith suites in the Thaba Putsoa and Mothae kimberlites generates revised upper mantle P-T arrays which refute the widely accepted advocacy by Finnerty and Boyd (1984, 1987) and Finnerty (1989) of an upper-mantle palaeogeotherm beneath northern Lesotho that is markedly inflected to a higher thermal gradient at the depths of derivation of the more chemically fertile, porphyroclastic textured, xenoliths.

