Introduction
Franksousaite, ideally PbCu(Se6+O4)(OH)2, is a new mineral species from the El Dragón mine, Antonio Quijarro Province, Potosí Department, Bolivia. It is named in honour of Mr. Francis (Frank) X. Sousa (born in 1951). Frank's interest in minerals began at age 9 and he started collecting minerals, especially Pb-bearing minerals, in his teenage years. He received his B.S. degree in geology from Oklahoma State University in 1973 and M.S degree in mineralogy, petrology and economic geology from the University of Arizona in 1980. Since joining the Tucson Gem and Mineral Society in 1976, Frank has served as recording secretary, property manager, collection manager, show floor setup manager and guest exhibit chair. In particular, he has been actively involved in youth education activities related to mineral collecting and identifications. Frank is a highly dedicated volunteer and takes great pride in it with his outstanding knowledge and experience in mineralogy. Since 2016, he has been a volunteer at the Arizona Sonora Desert Museum, the University of Arizona Mineral Museum and the University of Arizona Mineralogy Laboratory at the Department of Geosciences. In addition, Frank voluntarily taught several classes on geology and mineralogy to senior citizens through the University of Arizona's Osher Lifelong Learning Institute and on mineral identifications at Pima Community College in Tucson, Arizona. He has helped Boy Scouts earn the Geology Merit Badge and performed advisory judging for 4H youth rock-collection displays. One of Frank's great joys for mineral collecting is to gift specimens to both youths and adults and to share interesting geological stories behind them. The new mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA2021–096, Yang et al., Reference Yang, McGlasson, Gibbs and Downs2022). The co-type samples have been deposited at the University of Arizona Alfie Norville Gem and Mineral Museum (Catalogue # 22713) and the RRUFF Project (deposition # R210012) (http://rruff.info) (Lafuente et al., Reference Lafuente, Downs, Yang, Stone, Armbruster and Danisi2015). This paper describes the physical and chemical properties of franksousaite, and its crystal structure determined from single-crystal X-ray diffraction data, illustrating its structural relationships to the minerals of the linarite group.
Sample description and experimental methods
Occurrence, physical and chemical properties, and Raman spectra
Franksousaite was found on a specimen (Fig. 1) collected from the El Dragón mine (19°49′15″S, 65°55′0″W), Antonio Quijarro Province, Potosí Department, Bolivia. Associated minerals are Co-bearing krut'aite–penroseite, chalcomenite, schmiederite, olsacherite, phosgenite, anglesite, and cerussite. Detailed descriptions on the geology and mineralogy of the El Dragón mine have been given by Grundmann et al. (Reference Grundmann, Lehrberger and Schnorrer-Köhler1990, Reference Grundmann, Lehrberger and Schnorrer-Köhler2007) and Grundmann and Förster (Reference Grundmann and Förster2017). This mine exploited a telethermal deposit consisting of a single selenide vein hosted in sandstones and shales. The major ore mineral is krut'aite, CuSe2, varying in composition to penroseite, NiSe2. Subsequent solutions rich in Bi, Pb and Hg resulted in the crystallisation of minerals such as clausthalite, petrovicite, watkinsonite, and the recently described minerals eldragónite, Cu6BiSe4(Se2) (Paar et al., Reference Paar, Cooper, Moëlo, Stanley, Putz, Topa, Roberts, Stirling, Raith and Rowe2012), grundmannite, CuBiSe2 (Förster et al., Reference Förster, Bindi and Stanley2016), hansblockite, (Cu,Hg)(Bi,Pb)Se2 (Förster et al., Reference Förster, Bindi, Stanley and Grundmann2017), cerromojonite, CuPbBiSe3 (Förster et al., Reference Förster, Bindi, Grundmann and Stanley2018) and nickeltyrrellite, CuNi2Se4 (Förster et al., Reference Förster, Ma, Grundmann, Bindi and Stanley2019). Oxidation produced a wide range of secondary Se-bearing minerals, such as favreauite, PbBiCu6O4(SeO3)4(OH)⋅H2O (Mills et al., Reference Mills, Kampf, Housley, Christy, Thorne, Chen and Steele2014), alfredopetrovite, Al2(Se4+O3)3⋅6H2O (Kampf et al., Reference Kampf, Mills, Nash, Thorne and Favreau2016a) and the new mineral franksousaite, described herein.

Fig. 1. The specimen on which the new mineral franksousaite, indicated by the blue arrow, was found.
Franksousaite occurs as blue prismatic crystals included in colourless anglesite (Figs 2 and 3), which is on a matrix consisting of Co-bearing krut'aite–penroseite. Individual crystals of franksousaite are found up to 0.05 × 0.02 × 0.02 mm. The blueish anglesite crystal in Fig. 2 was picked out and broken into small pieces (Fig. 3) in order to extract franksousaite crystals for the necessary analyses and measurements. In total, four tiny franksousaite crystals were recovered, but they all have some anglesite attached to them. It is very difficult, if not entirely impossible, to completely separate anglesite from franksousaite while trying to maintain the largest crystal sizes for the analyses and measurements. In Fig. 3, crystal A was used for the electron microprobe analysis and crystal B for X-ray diffraction data collection and then Raman spectral measurement. The blue portion on the left side of crystal B in Fig. 3 is a single crystal of franksousaite and the colourless portion on the right side a single crystal of anglesite. Although the collected X-ray diffraction data were from both franksousaite and anglesite crystals, they can be separated easily for the structure analysis (see the Structure section below for more details). The black spot on the left side of crystal B in Fig. 3 was caused by burning during the Raman data collection with the 532-nm laser at the full power of 150 mW. Therefore, the Raman data were collected at the 50% power of 150 mW (see the Raman section below).

Fig. 2. A microscopic view of blue franksousaite enclosed in a large colourless anglesite crystal, making the whole anglesite crystal look blueish.

Fig. 3. Broken fragments of blue franksousaite crystals enclosed in the large colourless anglesite crystal in Fig. 2. Crystal A was used for the electron microprobe analysis. Crystal B was used for X-ray diffraction data collection and then Raman spectral measurement.
Franksousaite is blue in transmitted light, transparent with very pale blue streak and vitreous lustre. It is brittle and has a Mohs hardness of 2–2½. Cleavage could not be determined due to the small crystal size. By its structural analogue to linarite, it should have perfect cleavage on {100}. The density could not be measured due to the small crystals and intergrowth with anglesite. The calculated density is 5.64 g/cm3 on the basis of the empirical chemical formula and unit cell volume from single-crystal X-ray diffraction data. No optical data were measured due to the intergrowth with anglesite. The Gladstone–Dale relationship (Mandarino, Reference Mandarino1981) gives n = 1.88. Franksousaite is insoluble in water and hydrochloric acid.
The chemical composition was determined using a Cameca SX-100 electron microprobe (wavelength dispersive spectroscopy mode, 15 kV, 10 nA and a beam diameter of 2 μm). The standards used for the probe analysis are given in Table 1, along with the determined compositions (5 analysis points). The resultant chemical formula, calculated on the basis of 6 O atoms per formula unit (apfu) (from the structure determination), is Pb1.02Cu0.98[(Se0.84S0.17)Σ1.01O4)](OH)2, which can be simplified to PbCu[(Se,S)O4)](OH)2.
Table 1. Analytical chemical compositions (in wt.%) for franksousaite.

* Trace amounts of Si, Cr and Te were detected by energy dispersive spectroscopy, however they were below the measurement limits (<3σ) by WDS. The H2O content was added to achieve the ideal value of 2H apfu.
The Raman spectrum of franksousaite (Fig. 4) was collected on a randomly oriented crystal with a Thermo Almega microRaman system, using a solid-state laser with a wavelength of 532 nm at 50% of 150 mW power and a thermoelectric cooled CCD detector. The laser is partially polarised with 4 cm–1 resolution and a spot size of 1 μm.

Fig. 4. Raman spectra of franksousaite, linarite, munakataite and schmiederite.
X-ray crystallography
Both the powder and single-crystal X-ray diffraction data for franksousaite were collected on a Bruker APEX2 CCD X-ray diffractometer equipped with graphite-monochromatised MoKα radiation. Listed in Table 2 are the measured powder X-ray diffraction data. The unit-cell parameters obtained from the powder X-ray diffraction data using the program by Downs et al. (Reference Downs, Bartelmehs, Gibbs and Boisen1993) are: a = 9.823(7), b = 5.729(2), c = 4.751(3) Å, β = 102.78(8)° and V = 260.8(2) Å3.
Table 2. Powder X-ray diffraction data for franksousaite.
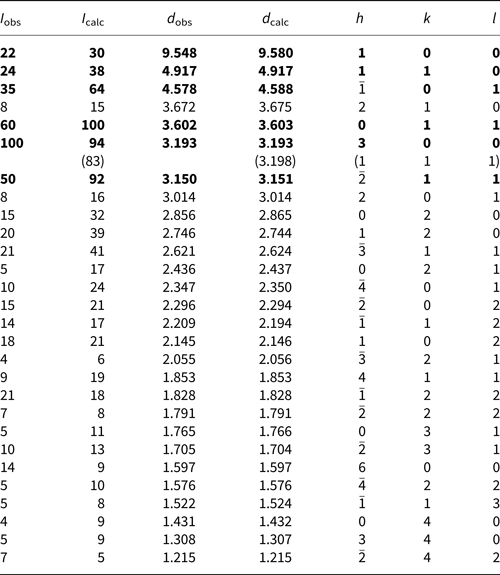
The strongest lines are given in bold.
Single-crystal X-ray diffraction data were collected from a fragment containing both franksousaite and anglesite single crystals (crystal B in Fig. 3) with frame widths of 0.5° in ω and 30 s counting time per frame. Using the Bruker software CELL_NOW (Sevvana et al., Reference Sevvana, Ruf, Isabel Usón, Sheldrick and Herbst-Irmere2019), the X-ray diffraction data of franksousaite and anglesite can be easily separated because the two crystals have different orientations and unit-cell dimensions (Fig. 5). The resultant intensity data for the two minerals are remarkably good and there are no obvious overlaps in the X-ray intensity data between the two minerals, as indicated by the subsequent structure refinements (R 1 = 0.020, wR 2 = 0.040, goodness-of-fit = 1.084 for franksousaite and R 1 = 0.0190, wR 2 = 0.031, goodness-of-fit = 1.072 for anglesite). All intensity data were corrected for X-ray absorption using the Bruker program SADABS (Bruker, 2001). The systematic absences of reflections suggest the possible space group P21 or P21/m for franksousaite and its structure was solved and refined using SHELX2018 (Sheldrick Reference Sheldrick2015a, Reference Sheldrick2015b) based on space group P21/m, because it produced better refinement statistics in terms of bond lengths and angles, atomic displacement parameters and R factors. All H atoms were located through the difference-Fourier syntheses. The structure refinement reveals that the Pb and Cu sites are fully filled by Pb and Cu, respectively, but the Se site has 18% Se substituted by S, consistent with the chemical composition determined from the electron microprobe analysis. All non-H atoms were refined anisotropically, whereas the two H atoms (H1 and H2) were refined isotropically. Final refinement statistics for franksousaite are listed in Table 3. Atomic coordinates and displacement parameters are given in Tables 4 and 5, respectively. Selected bond distances are presented in Table 6. The bond-valence sums were calculated using the parameters from Brown (Reference Brown2006) (Table 7). The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).

Fig. 5. A reciprocal space view of X-ray diffraction data collected from crystal B in Fig. 3. The green and red spots are for franksousaite and anglesite, respectively. Small black spots represent weak reflections [I < 3σ(I)]. The view is along the b axis of franksousaite. There are no obvious overlaps between the two lattice points.
Table 3. Comparison of crystallographic data and refinement results for minerals in the linarite group.

Table 4. Fractional atomic coordinates and equivalent isotropic displacement parameters (Å2) for franksousaite.

** Occupancies < 1 : Se = 0.820(4) and S = 0.180(4)
Table 5. Atomic displacement parameters (Å2) for franksousaite.

Table 6. Selected bond distances (Å) and angles (°) for franksousaite and linarite.

Note: T = Se for franksousaite and S for linarite.
Table 7. Bond-valence sums for franksousaite.

* The bond valence sum for Se was calculated based on (0.82Se6+ + 0.18S6+).
Crystal structure description and discussion
Franksousaite is the Se6+ analogue of linarite, PbCu(SO4)(OH)2 (Bachmann and Zemann, Reference Bachmann and Zemann1961; Araki, Reference Araki1962; Effenberger, Reference Effenberger1987; Schofield et al., Reference Schofield, Wilson, Knight and Kirk2009). Its structure consists of Jahn-Teller-distorted Cu2+O6 square bipyramids, which form chains along b by sharing trans edges across their square planes (Fig. 6). The chains are decorated by SeO4 tetrahedra, which bond to apical corners of adjacent bipyramids. The chains are linked to one another by hydrogen bonds to form layers parallel to (100), which are bound together by double layers of PbO8 and SeO4 polyhedra. The PbO8 polyhedron exhibits one-sided coordination typical of Pb2+ with a stereochemically active 6s 2 lone-electron-pair (Fig. 7). The major structural difference between franksousaite and linarite lies in the <Se–O> vs. <S–O> bond distances (Table 6), as the ionic radius of IVSe6+ (0.28 Å) is significantly larger than that of S6+ (0.12 Å) (Shannon Reference Shannon1976), accounting for the larger unit-cell volume for franksousaite.

Fig. 6. Crystal structure of franksousaite. Green elongated octahedra and yellow tetrahedra represent CuO6 and SeO4 groups, respectively. Large grey and small aqua spheres represent Pb and H atoms, respectively. Hydrogen bonds are indicated by grey lines.

Fig. 7. The coordination of a Pb atom by eight O atoms in franksousaite.
In addition to franksousaite and linarite, the linarite group also includes munakataite Pb2Cu2(Se4+O3)(SO4)(OH)4 and schmiederite Pb2Cu2(Se4+O3)(Se6+O4)(OH)4 (Table 3). Nevertheless, linarite and franksousaite are not isotypic, but show homeotypic relations with munakataite (Kampf et al., Reference Kampf, Mills and Housley2010) or schmiederite (Effenberger, Reference Effenberger1987), which have the c dimension doubled compared to linarite and franksousaite (Table 3), as a consequence of the ordering of Se4+O3 vs. SO4 in munakataite or Se4+O3 vs. Se6+O4 in schmiederite.
According to Raman spectroscopic studies on linarite (Buzgar et al., Reference Buzgar, Buzatu and Sanislav2009) and schmiederite (Frost and Keeffe, Reference Frost and Keeffe2008), as well as other hydrous materials containing (Se4+O3)2–, (Se6+O4)2–, and/or (SO4)2– (e.g. Wickleder et al., Reference Wickleder, Buchner, Wickleder, Sheik, Brunklaus and Eckert2004; Frost et al., Reference Frost, Weier, Reddy and Čejka2006; Djemel et al., Reference Djemel, Abdelhedi, Ktari and Dammak2013; Wolak et al., Reference Wolak, Pawlowski, Polomska and Pietraszko2013; Kasatkin et al., Reference Kasatkin, Plášil, Marty, Agakhanov, Belakovskiy and Lykova2014; Mills et al., Reference Mills, Kampf, Housley, Christy, Thorne, Chen and Steele2014; Kampf et al., Reference Kampf, Mills and Nash2016b), we made the following tentative assignments of major Raman bands for franksousaite. The weak peak at 3444 cm–1 is due to the O–H stretching vibrations in the OH groups. The small sharp bands at 932 and 1141 cm–1, resulting from the presence of a small amount of (SO4)2– substituting for (SeO4)2–, can be attributed to the S–O symmetric and antisymmetric stretching vibrations within the SO4 group, respectively. The strongest sharp band at 820 cm–1 and the weak band 882 cm–1 are ascribable to the Se–O symmetric and antisymmetric stretching modes, respectively, within the SeO4 groups, whereas those from 300 to 530 cm–1 originate from the O–Se–O (and O–S–O) bending vibrations within the SeO4 groups. The very weak bands below 300 cm–1 are associated mainly with the rotational and translational modes of SeO4/SO4 groups, as well as the Cu–O interactions and lattice vibrational modes.
The bond-valence sums indicate that O2 is noticeably under-bonded (1.74 valence units). This deficiency is compensated by the hydrogen bond, as it is engaged in the hydrogen bonding as an acceptor (Table 7). According to the correlation between νO–H and O—H⋅⋅⋅O distances for minerals (Libowitzky, Reference Libowitzky1999), the Raman band at 3444 cm–1 corresponds well with the O–O distances between 2.70 and 2.95 Å (O4—H1⋅⋅⋅O2 and O5—H2⋅⋅⋅O4).
For comparison, the Raman spectra of linarite (http://rruff.info/R060130), munakataite (http://rruff.info/R110006) and schmiederite (http://rruff.info/R100089), which are in the same mineral group as franksousaite, from the RRUFF Project are also plotted in Fig. 4. The spectral differences among the four minerals are obvious, especially in the range from 700 to 1000 cm–1, indicative of the presence or absence of (SeO3)2–, (Se6+O4)2– and/or (SO4)2– groups.
Acknowledgements
We are grateful for the constructive comments by Dr. Elena Zhitova and three other anonymous reviewers. This study was funded by the Feinglos family and Mr. Michael M. Scott.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.90
Competing interests
The authors declare none.
















