Published online by Cambridge University Press: 05 July 2018
Fluoro-sodic-ferropedrizite, ideally ANaBLi2C(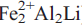 )TSi8O22WF2, is a new mineral of the amphibole group from the Sutlug River, Tuva Republic, Russia. It occurs at the endogenic contact of a Li-pegmatite with country rocks near to a diabase dyke and formed by reaction of the pegmatitic melt with the country rock. Fluoro-sodic-ferropedrizite occurs as prismatic to acicular crystals, ranging in length from 0.1–3 cm and widths of up to 50 μm. Crystals occur inparallel to sub-parallel aggregates up to 5 mm across ina matrix of calcite and plagioclase feldspar. Crystals are pale bluish-grey with a greyish-white streak.
)TSi8O22WF2, is a new mineral of the amphibole group from the Sutlug River, Tuva Republic, Russia. It occurs at the endogenic contact of a Li-pegmatite with country rocks near to a diabase dyke and formed by reaction of the pegmatitic melt with the country rock. Fluoro-sodic-ferropedrizite occurs as prismatic to acicular crystals, ranging in length from 0.1–3 cm and widths of up to 50 μm. Crystals occur inparallel to sub-parallel aggregates up to 5 mm across ina matrix of calcite and plagioclase feldspar. Crystals are pale bluish-grey with a greyish-white streak.
Fluoro-sodic-ferropedrizite is brittle, has a Mohs hardness of ~6 and a splintery fracture; it is non-fluorescent with perfect {110} cleavage, no observable parting, and has a calculated density of 3.116 g cm–3. In plane-polarized light, it is pleochroic, X = pale purple-grey, Y = light grey, Z = colourless; X ^ a = 71.2º (in β acute), Y || b, Z ^ c = 83.4º (in β obtuse). Fluoro-sodic-ferropedrizite is biaxial positive, α = 1.642(1), β = 1.644(1), γ = 1.652(1); 2V(obs) = 68.0(3)º, 2V(calc) = 56.4º. Fluoro-sodic-ferropedrizite is monoclinic, space group C2/m, a = 9.3720(4) Å, b = 17.6312(8) Å, c = 5.2732(3) Å, β = 102.247(4)º, V = 851.5(2) Å3, Z = 2. The strongest ten X-ray diffraction lines in the powder patternare (d in Å ,(I),(hkl)): 8.146,(10),(110); 2.686,(9),(151); 3.008,(8),(310); 4.430,(7),(021); 2.485,(6),(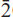 02); 3.383,(4),(131); 2.876,(3),(
02); 3.383,(4),(131); 2.876,(3),(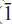 51,
51, 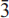 11); 2.199,(3),(
11); 2.199,(3),(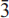 12); 4.030,(2),(111) and 3.795,(2),(
12); 4.030,(2),(111) and 3.795,(2),(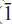 31). Analysis by a combination of electron microprobe and crystal-structure refinement gives SiO2 59.81, Al2O3 12.66, TiO2 0.09, FeO 10.32, MgO 5.56, MnO 0.73, ZnO 0.17, CaO 0.20, Na2O 2.81, Li2O 4.80, F 2.43, H2Ocalc 1.10, sum = 99.65 wt.%. The formula unit, calculated on the basis of 24(O,OH,F) is A(Na0.68)B(Li1.92Na0.05Ca0.03)C(
31). Analysis by a combination of electron microprobe and crystal-structure refinement gives SiO2 59.81, Al2O3 12.66, TiO2 0.09, FeO 10.32, MgO 5.56, MnO 0.73, ZnO 0.17, CaO 0.20, Na2O 2.81, Li2O 4.80, F 2.43, H2Ocalc 1.10, sum = 99.65 wt.%. The formula unit, calculated on the basis of 24(O,OH,F) is A(Na0.68)B(Li1.92Na0.05Ca0.03)C(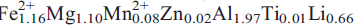 ) T(Si7.98Al0.02)O22W(F1.03OH0.97). Crystal-structure refinement shows Li to be completely ordered at the M(3) and M(4) sites. Fluoro-sodic-ferropedrizite, ideally ANaBLi2C(
) T(Si7.98Al0.02)O22W(F1.03OH0.97). Crystal-structure refinement shows Li to be completely ordered at the M(3) and M(4) sites. Fluoro-sodic-ferropedrizite, ideally ANaBLi2C(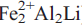 )TSi8O22WF2, is related to the theoretical end-member ‘sodic-pedrizite’, ANaBLi2C(Mg2Al2Li)TSi8O22W(OH)2, by the substitutions CFe2+ → CMg and WF → W(OH).
)TSi8O22WF2, is related to the theoretical end-member ‘sodic-pedrizite’, ANaBLi2C(Mg2Al2Li)TSi8O22W(OH)2, by the substitutions CFe2+ → CMg and WF → W(OH).