Article contents
Dwornikite, (Ni,Fe)SO4 · H2O, a member of the kieserite group from Minasragra, Peru
Published online by Cambridge University Press: 05 July 2018
Abstract
Dwornikite, (Ni1−xFex)SO4 · H2O is a member of the kieserite group, monoclinic with space group C2/c. Specimens from Minasragra, Peru with x ∼ 0.1 have a unit cell with a = 6.839(2), b = 7.582(2), c = 7.474(2) Å, and β = 117.85(2)°. The six strongest lines of the powder pattern are: 3.342 (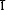 12, 100), 4.732 (110, 70), 3.024 (200, 70), 4.754 (
12, 100), 4.732 (110, 70), 3.024 (200, 70), 4.754 (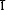 11, 50), 3.293 (021, 35), 2.491 (022, 35). The mineral occurs as fine grained white aggregates associated with vanadium sulphide ores containing patronite and bravoite, mixed with other oxidation products. New unit cell data for the synthetic end-member compounds NiSO4 · H2O and FeSO4 · H2O, and new X-ray powder data for retgersite (NiSO4 · 6H2O) are provided.
11, 50), 3.293 (021, 35), 2.491 (022, 35). The mineral occurs as fine grained white aggregates associated with vanadium sulphide ores containing patronite and bravoite, mixed with other oxidation products. New unit cell data for the synthetic end-member compounds NiSO4 · H2O and FeSO4 · H2O, and new X-ray powder data for retgersite (NiSO4 · 6H2O) are provided.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1982
References
- 4
- Cited by


