Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Siidra, Oleg I.
Nazarchuk, Evgeny V.
Agakhanov, Atali A.
and
Polekhovsky, Yury S.
2019.
Aleutite [Cu5O2](AsO4)(VO4)·(Cu0.5□0.5)Cl, a new complex salt-inclusion mineral with Cu2+substructure derived from a Kagome-net.
Mineralogical Magazine,
Vol. 83,
Issue. 6,
p.
847.
Britvin, S. N.
Pekov, I. V.
Yapaskurt, V. O.
Koshlyakova, N. N.
Göttlicher, J.
Krivovichev, S. V.
Turchkova, A. G.
and
Sidorov, E. G.
2020.
Polyoxometalate chemistry at volcanoes: discovery of a novel class of polyoxocuprate nanoclusters in fumarolic minerals.
Scientific Reports,
Vol. 10,
Issue. 1,
Pekov, Igor V.
Zubkova, Natalia V.
Yapaskurt, Vasiliy O.
Polekhovsky, Yury S.
Britvin, Sergey N.
Turchkova, Anna G.
Sidorov, Evgeny G.
and
Pushcharovsky, Dmitry Yu.
2020.
Kainotropite, Cu4Fe3+O2(V2O7)(VO4), a new mineral with a complex vanadate anion from fumarolic exhalations of the Tolbachik volcano, Kamchatka, Russia.
The Canadian Mineralogist,
Vol. 58,
Issue. 2,
p.
155.
Pekov, I. V.
Zubkova, N. V.
Yapaskurt, V. O.
Koshlyakova, N. N.
Turchkova, A. G.
Sidorov, E. G.
and
Pushcharovsky, D. Yu.
2020.
Polymorphism and Isomorphic Substitutions in the $${\text{Cu}}_{3}^{{2 + }}$$(T
5+O4)2 Natural System with T = As, V, or P.
Geology of Ore Deposits,
Vol. 62,
Issue. 8,
p.
803.
Kornyakov, Ilya V.
Vladimirova, Victoria A.
Siidra, Oleg I.
and
Krivovichev, Sergey V.
2021.
Expanding the Averievite Family, (MX)Cu5O2(T5+O4)2 (T5+ = P, V; M = K, Rb, Cs, Cu; X = Cl, Br): Synthesis and Single-Crystal X-ray Diffraction Study.
Molecules,
Vol. 26,
Issue. 7,
p.
1833.
Zhitova, E. S.
Anikin, L. P.
Sergeeva, A. V.
Ismagilova, R. M.
Rashidov, V. A.
Chubarov, V. M.
and
Kupchinenko, A. N.
2021.
Volborthite Occurrence at the Alaid Volcano (Atlasov Island, Kuril Islands, Russia).
Geology of Ore Deposits,
Vol. 63,
Issue. 7,
p.
735.
Siidra, Oleg I.
Borisov, Artem S.
Charkin, Dmitri O.
Depmeier, Wulf
and
Platonova, Natalia V.
2021.
Evolution of fumarolic anhydrous copper sulfate minerals during successive hydration/dehydration.
Mineralogical Magazine,
Vol. 85,
Issue. 2,
p.
262.
Borisov, Artem S.
Siidra, Oleg I.
Kovrugin, Vadim M.
Golov, Andrey A.
Depmeier, Wulf
Nazarchuk, Evgeny V.
and
Holzheid, Astrid
2021.
Expanding the family of mineral-like anhydrous alkali copper sulfate framework structures: new phases, topological analysis and evaluation of ion migration potentialities.
Journal of Applied Crystallography,
Vol. 54,
Issue. 1,
p.
237.
Kornyakov, Ilya V.
and
Krivovichev, Sergey V.
2022.
Na2Cu+[Cu2+
3O](AsO4)2Cl and Cu3[Cu3O]2(PO4)4Cl2: two new structure types based upon chains of oxocentered tetrahedra.
Zeitschrift für Kristallographie - Crystalline Materials,
Vol. 237,
Issue. 8-9,
p.
343.
Shablinskii, Andrey P.
Avdontceva, Margarita S.
Vergasova, Lidiya P.
Filatov, Stanislav K.
Avdontseva, Evgenia Yu.
Povolotskiy, Alexey V.
Moskaleva, Svetlana V.
Kargopoltsev, Anatoly A.
Britvin, Sergey N.
and
Shorets, Olga U.
2022.
Medvedevite, KMn2+V5+2O6Cl⋅2H2O, a new fumarolic mineral from the Tolbachik fissure eruption 2012–2013, Kamchatka Peninsula, Russia.
Mineralogical Magazine,
Vol. 86,
Issue. 3,
p.
478.
Ginga, Victoria A.
Siidra, Oleg I.
Firsova, Vera A.
Charkin, Dmitri O.
and
Ugolkov, Valery L.
2022.
Phase evolution and temperature-dependent behavior of averievite, Cu5O2(VO4)2(CuCl) and yaroshevskite, Cu9O2(VO4)4Cl2.
Physics and Chemistry of Minerals,
Vol. 49,
Issue. 9,
Ginga, Victoria A.
Siidra, Oleg I.
Breitner, Franziska
Jesche, Anton
and
Tsirlin, Alexander A.
2022.
Chemical Vapor Transport Synthesis of Cu(VO)2(AsO4)2 With Two Distinct Spin-1/2 Magnetic Ions.
Inorganic Chemistry,
Vol. 61,
Issue. 42,
p.
16539.
Kiriukhina, Galina
Yakubovich, Olga
Verchenko, Polina
Volkov, Anatoly
Shvanskaya, Larisa
Dimitrova, Olga
and
Simonov, Sergey
2023.
Mineral-like Synthetic Compounds Stabilized under Hydrothermal Conditions: X-ray Diffraction Study and Comparative Crystal Chemistry.
Minerals,
Vol. 14,
Issue. 1,
p.
46.
Ginga, Victoria A.
Siidra, Oleg I.
Tsirlin, Alexander A.
and
Setzer, Annette
2024.
Assembling the Puzzle of Coparsite Polymorphism: Synthesis, Thermal Expansion, and Quantum Magnetism of α- and β-Cu4O2(VO4)Cl.
Inorganic Chemistry,
Vol. 63,
Issue. 52,
p.
24573.
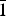 $\bar{1}$, a = 6.332(3), b = 8.204(4), c = 15.562(8) Å, α = 90.498(8), β = 97.173(7), γ = 90.896(13)°, V = 801.9(7) Å3 and R1 = 0.057. The eight strongest lines of the X-ray powder diffraction pattern are (d, Å (I)(hkl): (15.4396)(18)(00
$\bar{1}$, a = 6.332(3), b = 8.204(4), c = 15.562(8) Å, α = 90.498(8), β = 97.173(7), γ = 90.896(13)°, V = 801.9(7) Å3 and R1 = 0.057. The eight strongest lines of the X-ray powder diffraction pattern are (d, Å (I)(hkl): (15.4396)(18)(00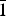 $\bar{1}$), (7.2762)(27)(0
$\bar{1}$), (7.2762)(27)(0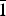 $\bar{1}$1), (5.5957)(43)(012), (4.8571)(33)(
$\bar{1}$1), (5.5957)(43)(012), (4.8571)(33)(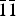 $\bar{1}\bar{1}$1), (3.1929) (29)(023), (2.7915)(30)(202), (2.5645)(21)(032), (2.5220)(100)(1
$\bar{1}\bar{1}$1), (3.1929) (29)(023), (2.7915)(30)(202), (2.5645)(21)(032), (2.5220)(100)(1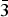 $\bar{3}$0), (2.4906)(18)(130) and (2.3267)(71)(2
$\bar{3}$0), (2.4906)(18)(130) and (2.3267)(71)(2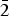 $\bar{2}$2). The chemical composition determined by electron-microprobe analysis is (wt.%): CuO 60.87, ZnO 0.50, FeO 0.36, V2O5 19.85, As2O5 6.96, SO3 0.44, MoO3 1.41, SiO2 0.20, P2O5 0.22, Cl 10.66, –O = Cl2 2.41, total 99.06. The empirical formula calculated on the basis of 17 anions per formula unit is (Cu7.72Zn0.06Fe0.05)Σ7.83(V2.20As0.61Mo0.10S0.06P0.03Si0.03)Σ3.03O13.96Cl3.04.
$\bar{2}$2). The chemical composition determined by electron-microprobe analysis is (wt.%): CuO 60.87, ZnO 0.50, FeO 0.36, V2O5 19.85, As2O5 6.96, SO3 0.44, MoO3 1.41, SiO2 0.20, P2O5 0.22, Cl 10.66, –O = Cl2 2.41, total 99.06. The empirical formula calculated on the basis of 17 anions per formula unit is (Cu7.72Zn0.06Fe0.05)Σ7.83(V2.20As0.61Mo0.10S0.06P0.03Si0.03)Σ3.03O13.96Cl3.04.