Published online by Cambridge University Press: 05 July 2018
Edwardsite, Cu3Cd2(SO4)2(OH)6·4H2O, is a new mineral from the Block 14 Opencut, Broken Hill, New South Wales, Australia. It occurs as druses of tabular and bladed crystals up to 0.06 mm in size, associated with niedermayrite and christelite. Edwardsite is pale blue, transparent with vitreous lustre and has excellent cleavage parallel to {100}. Density was not measured but the calculated density, from the empirical formula, is 3.53 g cm–3 and the Mohs hardness is ∼3. Optically, it is biaxial negative with α ∼ 1.74, β = 1.762(4), γ ∼ 1.77 and 2Vcalc. ∼ +62°. The optical orientation is X = b, Y ∼ a, Z ∼ c. Electron microprobe analysis gave (wt.%): CdO 32.43, CuO 28.06, ZnO 2.26, FeO 0.08, SO3 20.35, H2Ocalc. (from crystal-structure analysis) 14.14, totalling 99.32. The empirical formula, calculated on the basis of 18 oxygen atoms is Cu2.77Cd1.98Zn0.22Fe0.01(SO4)2.00(OH)5.95·4.06H2O. Edwardsite is monoclinic, space group P21/c, with a = 10.863(2) Å, b = 13.129(3) Å, c = 11.169(2) Å, β = 113.04(3)°, V = 1465.9(5) Å3 (single-crystal data) and Z = 4. The eight strongest lines in the powder diffraction pattern are [d (Å), (I/I0), (hkl)]: 9.991, (90), (100); 5.001, (90), (200, 21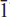 ); 4.591, (45), (20
); 4.591, (45), (20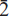 ); 3.332, (60), (300, 032); 3.005, (30), (0
); 3.332, (60), (300, 032); 3.005, (30), (0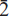 3); 2.824, (40), (
3); 2.824, (40), (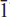
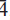 2); 2.769, (55), (20
2); 2.769, (55), (20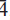 , 042, 10
, 042, 10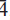 ); 2.670, (45), (2
); 2.670, (45), (2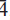
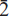 ). The crystal structure was determined by direct methods and refined to R1 = 3.21% using 1904 observed reflections with Fo > 4σ(Fo) collected using synchrotron X-ray radiation (λ = 0.773418 Å). The structure is based on infinite sheets of edge-sharing Cuϕ6 (ϕ: O2–, OH) octahedra and Cdϕ7 (ϕ: O2–, H2O) polyhedra parallel to (100). The sheets are decorated on both sides by corner-sharing (SO4) tetrahedra, which also corner-link to isolated Cdϕ6 octahedra, thus connecting adjacent sheets. Moderate-strong to weak hydrogen bonding provides additional linkage between sheets.
). The crystal structure was determined by direct methods and refined to R1 = 3.21% using 1904 observed reflections with Fo > 4σ(Fo) collected using synchrotron X-ray radiation (λ = 0.773418 Å). The structure is based on infinite sheets of edge-sharing Cuϕ6 (ϕ: O2–, OH) octahedra and Cdϕ7 (ϕ: O2–, H2O) polyhedra parallel to (100). The sheets are decorated on both sides by corner-sharing (SO4) tetrahedra, which also corner-link to isolated Cdϕ6 octahedra, thus connecting adjacent sheets. Moderate-strong to weak hydrogen bonding provides additional linkage between sheets.