Published online by Cambridge University Press: 05 July 2018
Debattistiite, ideally Ag9Hg0.5As6S12Te2, is a new mineral (IMA-CNMNC 2011-098) from the Lengenbach quarry in the Binn Valley, Valais, Switzerland. It occurs as very rare tabular euhedral crystals up to 150 μm across in cavities in dolomitic marble, associated with realgar, rutile, trechmannite and hutchinsonite. Debattistiite is opaque with a metallic lustre and a grey streak. It is brittle; the Vickers hardness (VHN25) is 80 kg mm–2 (range: 65–94), corresponding to a Mohs hardness of 2–2½. In reflected light debattistiite is dark grey, highly bireflectant and weakly pleochroic from dark grey to a slightly greenish grey. Between crossed polars it is highly anisotropic with brownish to blue rotation tints. Internal reflections are absent. Reflectance percentages for the four COM wavelengths (Rmin and Rmax) are 27.2, 34.5 (471.1 nm), 25.5, 31.0 (548.3 nm), 22.9, 28.4 (586.6 nm), and 20.1, 25.2 (652.3 nm), respectively.
Debattistiite is triclinic, space group P1, with a = 7.832(5), b = 8.606(4), c = 10.755(5) Å, α = 95.563(9), β = 95.880(5), γ = 116.79(4)°, V = 635.3(6) Å3 and Z = 1. The crystal structure [R1 = 0.0826 for 795 reflections with I > 2σ(I)] consists of corner-sharing AsS3 pyramids forming three-membered distorted rings linked by Ag atoms in triangular or tetrahedral coordination.
The five strongest powder-diffraction lines [d in Å (I/I0) (hkl)] are as follows: 10.56 (6) (001); 3.301 (5) ( 12); 2.991 (4) (2
12); 2.991 (4) (2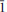 2); 2.742 (
2); 2.742 (
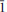 1) and 2.733 (10) (
1) and 2.733 (10) ( 30). A mean of nine electron microprobe analyses gave: Ag 44.88, Hg 4.49, As 20.77, S 17.72, Te 11.82; total 99.68 wt.%, which corresponds to Ag9.02Hg0.49As6.012S11.98Te2.01, on the basis of 29.5 atoms. The new mineral is named for Luca De Battisti, a systematic mineralogist and expert on the minerals of Lengenbach quarry.
30). A mean of nine electron microprobe analyses gave: Ag 44.88, Hg 4.49, As 20.77, S 17.72, Te 11.82; total 99.68 wt.%, which corresponds to Ag9.02Hg0.49As6.012S11.98Te2.01, on the basis of 29.5 atoms. The new mineral is named for Luca De Battisti, a systematic mineralogist and expert on the minerals of Lengenbach quarry.