Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Scordari, F.
and
Scandale, E.
1980.
Merohedric twinning in a sulphate related to Maus' salt and metavoltine *.
Zeitschrift für Kristallographie - Crystalline Materials,
Vol. 151,
Issue. 3-4,
p.
325.
Scordari, F.
1980.
Structural considerations of some natural and artificial alkali iron hydrated sulphates.
Mineralogical Magazine,
Vol. 43,
Issue. 329,
p.
669.
Scordari, F.
1981.
Sideronatrite: A mineral with a {Fe2(SO4)4(OH)2} guildite type chain?.
TMPM Tschermaks Mineralogische und Petrographische Mitteilungen,
Vol. 28,
Issue. 4,
p.
315.
Scordari, F.
1983.
Solid-state reactions in “salt X”.
Zeitschrift für Kristallographie - Crystalline Materials,
Vol. 163,
Issue. 1-2,
p.
31.
Dick, Stefan
Goβner, Ursula
Weiβ, Armin
Robl, Christian
Groβmann, Gisbert
Ohms, Gisela
and
Zeiske, Thomas
1998.
Taranakite — the mineral with the longest crystallographic axis.
Inorganica Chimica Acta,
Vol. 269,
Issue. 1,
p.
47.
Krivovichev, Sergey V.
2004.
Combinatorial topology of salts of inorganic oxoacids: zero-, one- and two-dimensional units with corner-sharing between coordination polyhedra.
Crystallography Reviews,
Vol. 10,
Issue. 3,
p.
185.
Villars, P.
Cenzual, K.
Daams, J.
Gladyshevskii, R.
Shcherban, O.
Dubenskyy, V.
Kuprysyuk, V.
and
Savysyuk, I.
2010.
Structure Types. Part 9: Space Groups (148) R-3 - (141) I41/amd.
Vol. 43A9,
Issue. ,
p.
316.
Kaufman, Elizabeth A.
Zeller, Matthias
and
Norquist, Alexander J.
2010.
A Slow Leak Synthetic Route to Organically Templated Gallium Sulfates.
Crystal Growth & Design,
Vol. 10,
Issue. 10,
p.
4656.
Han, Young-Soo
Hyun, Sung Pil
Jeong, Hoon Y.
and
Hayes, Kim F.
2012.
Kinetic study of cis-dichloroethylene (cis-DCE) and vinyl chloride (VC) dechlorination using green rusts formed under varying conditions.
Water Research,
Vol. 46,
Issue. 19,
p.
6339.
Demartin, F.
Castellano, C.
and
Campostrini, I.
2013.
Aluminopyracmonite, (NH4)3Al(SO4)3, a new ammonium aluminium sulfate from La Fossa crater, Vulcano, Aeolian Islands, Italy.
Mineralogical Magazine,
Vol. 77,
Issue. 4,
p.
443.
Hawthorne, F. C.
2014.
The structure hierarchy hypothesis.
Mineralogical Magazine,
Vol. 78,
Issue. 4,
p.
957.
Marshall, Kayleigh L.
and
Weller, Mark T.
2014.
Synthesis of Titanium Fluorophosphates and Fluorosulfates from Hexafluorotitanic Acid.
Zeitschrift für anorganische und allgemeine Chemie,
Vol. 640,
Issue. 14,
p.
2766.
Kampf, Anthony R.
Mills, Stuart J.
Nash, Barbara P.
Dini, Maurizio
and
Molina Donoso, Arturo A.
2017.
Currierite, Na4Ca3MgAl4(AsO3OH)12·9H2O, a new acid arsenate with ferrinatrite-like heteropolyhedral chains from the Torrecillas mine, Iquique Province, Chile.
Mineralogical Magazine,
Vol. 81,
Issue. 5,
p.
1141.
Ventruti, Gennaro
Ventura, Giancarlo Della
Lacalamita, Maria
Sbroscia, Marco
Sodo, Armida
Plaisier, Jasper R.
Cinque, Gianfelice
and
Schingaro, Emanuela
2019.
Crystal-chemistry and vibrational spectroscopy of ferrinatrite, Na3[Fe(SO4)3]·3H2O, and its high-temperature decomposition.
Physics and Chemistry of Minerals,
Vol. 46,
Issue. 2,
p.
119.
Yang, Zhuming
and
Giester, Gerald
2019.
Structure refinement and hydrogen bonding of ferrinatrite, Na3Fe(SO4)3⋅3H2O.
Mineralogy and Petrology,
Vol. 113,
Issue. 4,
p.
555.
Zolotarev, Andrey A.
Krivovichev, Sergey V.
Avdontceva, Margarita S.
Shilovskikh, Vladimir V.
Rassomakhin, Mikhail A.
Yapaskurt, Vasiliy O.
and
Pekov, Igor V.
2020.
Crystal Chemistry of Alkali–Aluminum–Iron Sulfates from the Burnt Mine Dumps of the Chelyabinsk Coal Basin, South Urals, Russia.
Crystals,
Vol. 10,
Issue. 11,
p.
1062.
Krivovichev, S. V.
2023.
Hydrogen Bonding in Parascorodite and Relative Stability of Fe(AsO4)⋅2H2O Polymorphs.
Geology of Ore Deposits,
Vol. 65,
Issue. 7,
p.
765.
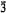 , with a = 15·566(5), c = 8·69(1) Å, and Z = 6. The crystal structure was solved by three-dimensional Patterson and Fourier syntheses, and refined by least squares employing 2378 independent reflexions to a final R value of 0·068. The iron ions occupy special positions and are surrounded octahedrally by oxygen atoms. Fe3+O6 octahedra and SO4, tetrahedra are linked together to form infinite chains of Fe-O-S linkages in the [0001] direction. These chains are linked to each other by [NaO5(H2O)2] polyhedra and probably by hydrogen bonds. The topology of the arrangement is the same as that of the hypothetical P312 structure proposed by Moore and Araki (1974).
, with a = 15·566(5), c = 8·69(1) Å, and Z = 6. The crystal structure was solved by three-dimensional Patterson and Fourier syntheses, and refined by least squares employing 2378 independent reflexions to a final R value of 0·068. The iron ions occupy special positions and are surrounded octahedrally by oxygen atoms. Fe3+O6 octahedra and SO4, tetrahedra are linked together to form infinite chains of Fe-O-S linkages in the [0001] direction. These chains are linked to each other by [NaO5(H2O)2] polyhedra and probably by hydrogen bonds. The topology of the arrangement is the same as that of the hypothetical P312 structure proposed by Moore and Araki (1974).

