Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Zhitova, Elena S.
Krivovichev, Sergey V.
Pekov, Igor V.
and
Yapaskurt, Vasiliy O.
2019.
Crystal Chemistry of Chlormagaluminite, Mg4Al2(OH)12Cl2(H2O)2, a Natural Layered Double Hydroxide.
Minerals,
Vol. 9,
Issue. 4,
p.
221.
Zhitova, Elena S.
Pekov, Igor V.
Chaikovskiy, Ilya I.
Chirkova, Elena P.
Yapaskurt, Vasiliy O.
Bychkova, Yana V.
Belakovskiy, Dmitry I.
Chukanov, Nikita V.
Zubkova, Natalia V.
Krivovichev, Sergey V.
and
Bocharov, Vladimir N.
2019.
Dritsite, Li2Al4(OH)12Cl2·3H2O, a New Gibbsite-Based Hydrotalcite Supergroup Mineral.
Minerals,
Vol. 9,
Issue. 8,
p.
492.
Monzó, Fuensanta
Caparrós, Ana
Pérez-Pérez, Diego
Arribas, Alejandro
and
Pamies, Ramón
2019.
Synthesis and Characterization of New Layered Double Hydroxide-Polyolefin Film Nanocomposites with Special Optical Properties.
Materials,
Vol. 12,
Issue. 21,
p.
3580.
Rojas, Hugo A.
López, Viviana P.
Brijaldo, Maria H.
Mancipe, Sonia
Martínez, José J.
Gómez-Cortés, Antonio
Araiza, Daniel G.
and
Díaz, Gabriela
2020.
Effect of boron on the surface properties of nickel supported on hydrotalcite-type mixed oxides in methanol decomposition.
Molecular Catalysis,
Vol. 498,
Issue. ,
p.
111262.
Karpenko, Vladimir Yu.
Zhitova, Elena S.
Pautov, Leonid A.
Agakhanov, Atali A.
Siidra, Oleg I.
Krzhizhanovskaya, Maria G.
Rassulov, Victor A.
and
Bocharov, Vladimir N.
2020.
Akopovaite, Li2Al4(OH)12(CO3)(H2O)3, a new Li member of the hydrotalcite supergroup from Turkestan Range, Kyrgyzstan.
Mineralogical Magazine,
Vol. 84,
Issue. 2,
p.
301.
Zhitova, E.S.
Pekov, I.V.
Chukanov, N.V.
Yapaskurt, V.O.
and
Bocharov, V.N.
2020.
Minerals of the System Stichtite–Pyroaurite–Iowaite–Woodallite from Serpentinites of the Terekta Ridge (Gorny Altai, Russia).
Russian Geology and Geophysics,
Vol. 61,
Issue. 1,
p.
36.
Farhat, Hani
Taviot-Gueho, Christine
Monier, Guillaume
Briois, Valérie
Forano, Claude
and
Mousty, Christine
2020.
Insights into the Structure and the Electrochemical Reactivity of Cobalt-Manganese Layered Double Hydroxides: Application to H2O2 Sensing.
The Journal of Physical Chemistry C,
Vol. 124,
Issue. 28,
p.
15585.
Zhitova, Elena S.
Greenwell, H. Chris
Krzhizhanovskaya, Mariya G.
Apperley, David C.
Pekov, Igor V.
and
Yakovenchuk, Victor N.
2020.
Thermal Evolution of Natural Layered Double Hydroxides: Insight from Quintinite, Hydrotalcite, Stichtite, and Iowaite as Reference Samples for CO3- and Cl-Members of the Hydrotalcite Supergroup.
Minerals,
Vol. 10,
Issue. 11,
p.
961.
Nielsen, Ulla Gro
2021.
Vol. 104,
Issue. ,
p.
75.
Gabalcová, Zuzana
Gogola, Peter
Kusý, Martin
and
Suchánek, Henrich
2021.
The Effect of Sn Addition on Zn-Al-Mg Alloy; Part II: Corrosion Behaviour.
Materials,
Vol. 14,
Issue. 18,
p.
5290.
Zhitova, Elena S.
Chukanov, Nikita V.
Jonsson, Erik
Pekov, Igor V.
Belakovskiy, Dmitry I.
Vigasina, Marina F.
Zubkova, Natalia V.
Van, Konstantin V.
and
Britvin, Sergey N.
2021.
Erssonite, CaMg7Fe3+2(OH)18(SO4)2⋅12H2O, a new hydrotalcite-supergroup mineral from Långban, Sweden.
Mineralogical Magazine,
Vol. 85,
Issue. 5,
p.
817.
Karcz, Robert
Napruszewska, Bogna D.
Michalik, Alicja
Kryściak-Czerwenka, Joanna
Duraczyńska, Dorota
and
Serwicka, Ewa M.
2021.
Fine Crystalline Mg-Al Hydrotalcites as Catalysts for Baeyer-Villiger Oxidation of Cyclohexanone with H2O2.
Catalysts,
Vol. 11,
Issue. 12,
p.
1493.
Yadav, Dileep Kumar
Uma, Sitharaman
and
Nagarajan, Rajamani
2022.
Microwave-assisted synthesis of ternary Li-M-Al LDHs (M = Mg, Co, Ni, Cu, Zn, and Cd) and examining their use in phenol oxidation.
Applied Clay Science,
Vol. 228,
Issue. ,
p.
106655.
Nestroinaia, Olga V.
Ryltsova, Irina G.
Yaprintsev, Maksim N.
Nakisko, Evgeniya Yu.
Seliverstov, Evgeniy S.
and
Lebedeva, Olga E.
2022.
Sorption of Congo Red anionic dye on natural hydrotalcite and stichtite: kinetics and equilibrium.
Clay Minerals,
Vol. 57,
Issue. 2,
p.
105.
Karcz, Robert
Napruszewska, Bogna D.
Walczyk, Anna
Kryściak-Czerwenka, Joanna
Duraczyńska, Dorota
Płaziński, Wojciech
and
Serwicka, Ewa M.
2022.
Comparative Physicochemical and Catalytic Study of Nanocrystalline Mg-Al Hydrotalcites Precipitated with Inorganic and Organic Bases.
Nanomaterials,
Vol. 12,
Issue. 16,
p.
2775.
Liu, Tao
Li, Shaohua
Chen, Yuxuan
Brouwers, H.J.H.
and
Yu, Qingliang
2023.
In-situ formation of layered double hydroxides in MgO–NaAlO2-activated GGBS / MSWI BA: Impact of Mg2+ on reaction mechanism and leaching behavior.
Cement and Concrete Composites,
Vol. 140,
Issue. ,
p.
105114.
Yu, Xiankun
Sun, Qi
Tian, Jingchen
Wan, Jie
Liu, Yanjun
Wang, Xiaoli
Kan, Jianfei
Yang, Xiaojun
and
Wu, Gongde
2023.
Construction of Supported MnOx/MgAl Hydrotalcite Catalysts and Their Highly Efficient Catalytic Performance for Low-Temperature Formaldehyde Removal.
Catalysts,
Vol. 13,
Issue. 9,
p.
1283.
Zhitova, Elena S.
Chukanov, Nikita V.
Pekov, Igor V.
Zolotarev, Andrey A.
Shilovskikh, Vladimir V.
and
Bocharov, Vladimir N.
2023.
Crystal chemistry of iowaite, Mg6FeIII2(OH)16Cl2 × 4H2O, a natural layered double hydroxide.
Applied Clay Science,
Vol. 243,
Issue. ,
p.
107070.
Terzi, Carolina Machado
dos Santos, Everton Henrique
Carvalho, Charles
Prevot, Vanessa
Wypych, Fernando
Forano, Claude
and
Nakagaki, Shirley
2023.
MgAl and ZnAl layered double hydroxides modified with molybdate and tungstate anions as catalysts for oxidation of cyclohexane.
Catalysis Today,
Vol. 422,
Issue. ,
p.
114221.
Zhitova, Elena S.
Sheveleva, Rezeda M.
Kasatkin, Anatoly V.
Zolotarev, Andrey A.
Bocharov, Vladimir N.
Kupchinenko, Anastasia N.
and
Belakovsky, Dmitry I.
2023.
Crystal Structure of Hydrotalcite Group Mineral—Desautelsite, Mg6MnIII2(OH)16(CO3)·4H2O, and Relationship between Cation Size and In-Plane Unit Cell Parameter.
Symmetry,
Vol. 15,
Issue. 5,
p.
1029.
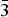 $ {\bar 3} $m on three crystals (two data collections at 290 K and one at 120 K). The unit-cell parameters are as follows (290/290/120 K): a = 3.0728(9)/3.0626(3)/3.0617(4), c = 23.326(9)/23.313(3)/23.203(3) Å and V = 190.7(1)/189.37(4)/188.36(4) Å3. The crystal structures were refined on the basis of 304/150/101 reflections to R1 = 0.075/0.041/0.038. Hydrotalcite-2H crystallises in the P63/mmc space group; unit-cell parameters for two crystals are (data collection at 290 K and 93 K): a = 3.046(1)/3.0521(9), c = 15.447(6)/15.439(4) Å, V = 124.39(8)/124.55(8) Å3. The crystal structures were refined on the basis of 160/142 reflections to R1 = 0.077/0.059. This paper reports the first single-crystal structure data on hydrotalcite. Hydrotalcite distribution in Nature, diagnostic features, polytypism, interlayer topology and localisation of M2+–M3+ cations within metal hydroxide layers are discussed.
$ {\bar 3} $m on three crystals (two data collections at 290 K and one at 120 K). The unit-cell parameters are as follows (290/290/120 K): a = 3.0728(9)/3.0626(3)/3.0617(4), c = 23.326(9)/23.313(3)/23.203(3) Å and V = 190.7(1)/189.37(4)/188.36(4) Å3. The crystal structures were refined on the basis of 304/150/101 reflections to R1 = 0.075/0.041/0.038. Hydrotalcite-2H crystallises in the P63/mmc space group; unit-cell parameters for two crystals are (data collection at 290 K and 93 K): a = 3.046(1)/3.0521(9), c = 15.447(6)/15.439(4) Å, V = 124.39(8)/124.55(8) Å3. The crystal structures were refined on the basis of 160/142 reflections to R1 = 0.077/0.059. This paper reports the first single-crystal structure data on hydrotalcite. Hydrotalcite distribution in Nature, diagnostic features, polytypism, interlayer topology and localisation of M2+–M3+ cations within metal hydroxide layers are discussed.