Published online by Cambridge University Press: 02 January 2018
A crystallographic and chemical study of two 'elsmoreite' samples (previously described as 'ferritungstite') from the Hemerdon mine (now known as the Drakelands mine), Devon, United Kingdom has shown them to be two different polytypes of hydrokenoelsmoreite. Hydrokenoelsmoreite-3C(HKE-3C) crystallizes in space group 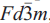 , with the unit-cell parameter a = 10.3065(3) Å. Hydrokenoelsmoreite-6R (HKE-6R) crystallizes in space group
, with the unit-cell parameter a = 10.3065(3) Å. Hydrokenoelsmoreite-6R (HKE-6R) crystallizes in space group 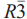 , with the unit-cell parameters a = 7.2882(2) Å and c = 35.7056(14)Å. Chemical analyses showed that both polytypes have Na and Fe/Al substitution giving the formulae: (Na0.28Ca0.04K0.02(H2O)0.20⁏1.46)∑2.00(W1.47Fe3+0.32Al0.21As5+0.01)∑2.00[O4.79(OH)1.21]∑6.00·(H2O)(3C) and (Na0.24Ca0.04K0.03(H2O)0.63⁏1.06)∑2.00(W1.42Fe3+0.49Al0.08As5+0.01)∑2.00[O4.65(OH)1.35]∑6.00·(H2O)(6R). The doubling of the unit cell in the 6R phase is due to ordering of Na and ( ,H2O) in the A site; no long-range ordering is observed between W and Fe/Al in the B site.
, with the unit-cell parameters a = 7.2882(2) Å and c = 35.7056(14)Å. Chemical analyses showed that both polytypes have Na and Fe/Al substitution giving the formulae: (Na0.28Ca0.04K0.02(H2O)0.20⁏1.46)∑2.00(W1.47Fe3+0.32Al0.21As5+0.01)∑2.00[O4.79(OH)1.21]∑6.00·(H2O)(3C) and (Na0.24Ca0.04K0.03(H2O)0.63⁏1.06)∑2.00(W1.42Fe3+0.49Al0.08As5+0.01)∑2.00[O4.65(OH)1.35]∑6.00·(H2O)(6R). The doubling of the unit cell in the 6R phase is due to ordering of Na and ( ,H2O) in the A site; no long-range ordering is observed between W and Fe/Al in the B site.
Current address: Queensland Museum, 122 Gerler Road, Hendra, Queensland 4011, and School of Earth Sciences, University of Queensland, St Lucia, Queensland 4072, Australia