Article contents
Calciodelrioite, Ca(VO3)2(H2O)4, the Ca analogue of delrioite, Sr(VO3)2(H2O)4
Published online by Cambridge University Press: 05 July 2018
Abstract
Calciodelrioite, ideally Ca(VO3)2(H2O)4, is a new mineral (IMA 2012-031) from the uraniumvanadium deposits of the eastern Colorado Plateau in the USA. The type locality is the West Sunday mine, Slick Rock district, San Miguel County, Colorado.The new mineral occurs on fracture surfaces in corvusite- and montroseite-impregnated sandstone and forms as a result of the oxidative alteration of these phases. At the West Sunday mine, calciodelrioite is associated with celestine, gypsum, huemulite, metarossite, pascoite and rossite. Themineral occurs as transparent colourless needles, bundles of tan to brown needles and star bursts of nearly black broad blades composed of tightly intergrown needles. Crystals are elongate and striated parallel to [100], exhibiting the prismatic forms {001} and {011} and having terminationspossibly composed of the forms {100} and {61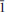 }. The mineral is transparent and has a white streak, subadamantine lustre, Mohs hardness of about 2½, brittle tenacity, irregular to splintery fracture, one perfect cleavage on {001} and possibly one or more additional cleavages parallel to [100].Calciodelrioite is soluble in water. The calculated density is 2.451 g cm– 3. It is optically biaxial (+) with α = 1.733(3), β = 1.775(3), γ = 1.825(3) (white light), 2Vmeas = 87.3(9)° and 2Vcalc = 87°. The optical orientationis X = b; Z ≈ a. No pleochroism was observed. Electronmicroprobe analyses of two calciodelrioite samples and type delrioite provided the empirical formulae (Ca0.88Sr0.07Na0.04K0.01)Σ1.00(V1.00O3)2(H2.01O)4,(Ca0.76Sr0.21Na0.01)Σ0.98(V1.00O3)2(H2.01O)4 and (Sr0.67Ca0.32)Σ0.99(V1.00O3)2(H2.00O)4, respectively.Calciodelrioite is monoclinic, I2/a, with unit-cell parameters a = 14.6389(10), b = 6.9591(4), c = 17.052(2) Å, β = 102.568(9)°, V = 1695.5(3) Å3 and Z = 8. The seven strongest lines in the X-ray powder diffractionpattern [listed as dobs Å (I)(hkl)] are as follows: 6.450(100)(011); 4.350(16)(013); 3.489(18)(020); 3.215(17)(022); 3.027(50)(multiple); 2.560(28)(
}. The mineral is transparent and has a white streak, subadamantine lustre, Mohs hardness of about 2½, brittle tenacity, irregular to splintery fracture, one perfect cleavage on {001} and possibly one or more additional cleavages parallel to [100].Calciodelrioite is soluble in water. The calculated density is 2.451 g cm– 3. It is optically biaxial (+) with α = 1.733(3), β = 1.775(3), γ = 1.825(3) (white light), 2Vmeas = 87.3(9)° and 2Vcalc = 87°. The optical orientationis X = b; Z ≈ a. No pleochroism was observed. Electronmicroprobe analyses of two calciodelrioite samples and type delrioite provided the empirical formulae (Ca0.88Sr0.07Na0.04K0.01)Σ1.00(V1.00O3)2(H2.01O)4,(Ca0.76Sr0.21Na0.01)Σ0.98(V1.00O3)2(H2.01O)4 and (Sr0.67Ca0.32)Σ0.99(V1.00O3)2(H2.00O)4, respectively.Calciodelrioite is monoclinic, I2/a, with unit-cell parameters a = 14.6389(10), b = 6.9591(4), c = 17.052(2) Å, β = 102.568(9)°, V = 1695.5(3) Å3 and Z = 8. The seven strongest lines in the X-ray powder diffractionpattern [listed as dobs Å (I)(hkl)] are as follows: 6.450(100)(011); 4.350(16)(013); 3.489(18)(020); 3.215(17)(022); 3.027(50)(multiple); 2.560(28)( 15,413); 1.786(18)(028). In the structure of calciodelrioite (refined to R1 = 3.14% for1216 Fo > 4σF), V5+O5 polyhedra link by sharing edges to form a zigzag divanadate [VO3] chain along a, similar to that in the structure of rossite. The chains are linked via bonds to Ca atoms, which also bond to H2Ogroups, yielding CaO3(H2O)6 polyhedra. The Ca polyhedra form a chain along b. Each of the two symmetrically independent VO5 polyhedra has two short vanadyl bonds and three long equatorial bonds. Calciodelrioite and delrioite are isostructuraland are the endmembers of the series Ca(VO3)2(H2O)4–Sr(VO3)2(H2O)4. Calciodelrioite is dimorphous with rossite, which has a similar structure; however, the smaller 8-coordinate Ca site in rossitedoes not accommodate Sr.
15,413); 1.786(18)(028). In the structure of calciodelrioite (refined to R1 = 3.14% for1216 Fo > 4σF), V5+O5 polyhedra link by sharing edges to form a zigzag divanadate [VO3] chain along a, similar to that in the structure of rossite. The chains are linked via bonds to Ca atoms, which also bond to H2Ogroups, yielding CaO3(H2O)6 polyhedra. The Ca polyhedra form a chain along b. Each of the two symmetrically independent VO5 polyhedra has two short vanadyl bonds and three long equatorial bonds. Calciodelrioite and delrioite are isostructuraland are the endmembers of the series Ca(VO3)2(H2O)4–Sr(VO3)2(H2O)4. Calciodelrioite is dimorphous with rossite, which has a similar structure; however, the smaller 8-coordinate Ca site in rossitedoes not accommodate Sr.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2016
References
- 3
- Cited by


