No CrossRef data available.
Published online by Cambridge University Press: 10 February 2023
Two new mineral species of the crichtonite group: botuobinskite, ideally SrFe2+(Ti4+12Cr3+6)Mg2[O36(OH)2] and mirnyite, ideally SrZr(Ti4+12Cr3+6)Mg2O38, occur as inclusions in mantle-derived Cr-pyrope xenocrysts from the Internatsionalnaya kimberlite pipe, Mirny field, Siberian craton. Botuobinskite forms needle- and blade-like acicular crystals up to 1 mm in length and up to 30 μm in diameter, a large platy inclusion (700 × 700 × 80 μm) and roughly isometric grains (up to 80 μm). Mirnyite occurs as needle-and blade-like elongated inclusions (up to 1 mm). Both minerals are jet-black, opaque and exhibit a metallic lustre. In plane-polarised reflected light, botuobinskite and mirnyite are greyish-white with a weak brownish tint. Between crossed polars, the new species show distinct anisotropy in shades of bluish grey to greenish-brown. Neither bireflectance nor pleochroism is observed. Calculated densities for botuobinskite and mirnyite are 4.3582(5) and 4.3867(3) gm/cm3, respectively. The crystal structures of botuobinskite and mirnyite have been refined (R = 0.0316 and 0.0285, respectively) from single crystal X-ray diffraction data. The minerals are trigonal, crystallise in the space group R$\bar{3}$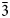 (No. 148) and are isostructural with other members of the crichtonite group. The unit cell parameters are a = 10.3644(8) Å, c = 20.6588(11) Å and V = 1921.9(2) Å3 for botuobinskite and a = 10.3734(8) Å, c = 20.6910 (12) Å and V = 1928.2(2) Å3 for mirnyite, with Z = 3 for both. The Raman spectra of the minerals show strong peaks at 133, 313 and 711 cm–1. Infrared spectroscopy data for botuobinskite indicates H–O stretching of the hydroxyl groups. Botuobinskite and mirnyite have been approved by the IMA–CNMNC under the numbers 2018-143a and 2018-144a, respectively. Botuobinskite and mirnyite are named after the Botuobinskaya exploration expedition and Mirny town, respectively. The minerals may be considered as crystal-chemical analogues of other crichtonite-group species occurring in the lithospheric mantle (i.e. loveringite, lindsleyite and mathiasite). Both species commonly occur in intimate association with Cr-pyrope as well as other peridotitic minerals and exert an important control on the partitioning of incompatible elements during mantle metasomatism.
(No. 148) and are isostructural with other members of the crichtonite group. The unit cell parameters are a = 10.3644(8) Å, c = 20.6588(11) Å and V = 1921.9(2) Å3 for botuobinskite and a = 10.3734(8) Å, c = 20.6910 (12) Å and V = 1928.2(2) Å3 for mirnyite, with Z = 3 for both. The Raman spectra of the minerals show strong peaks at 133, 313 and 711 cm–1. Infrared spectroscopy data for botuobinskite indicates H–O stretching of the hydroxyl groups. Botuobinskite and mirnyite have been approved by the IMA–CNMNC under the numbers 2018-143a and 2018-144a, respectively. Botuobinskite and mirnyite are named after the Botuobinskaya exploration expedition and Mirny town, respectively. The minerals may be considered as crystal-chemical analogues of other crichtonite-group species occurring in the lithospheric mantle (i.e. loveringite, lindsleyite and mathiasite). Both species commonly occur in intimate association with Cr-pyrope as well as other peridotitic minerals and exert an important control on the partitioning of incompatible elements during mantle metasomatism.
Associate Editor: Irina O Galuskina