Article contents
Bojarite, Cu3(N3C2H2)3(OH)Cl2⋅6H2O, a new mineral species with a microporous metal–organic framework from the guano deposit at Pabellón de Pica, Iquique Province, Chile
Published online by Cambridge University Press: 30 October 2020
Abstract
The new triazolate mineral bojarite (IMA2020-037), Cu3(N3C2H2)3(OH)Cl2⋅6H2O, is found in a guano deposit located at the Pabellón de Pica Mountain, Iquique Province, Tarapacá Region, Chile. Associated minerals are salammoniac, halite, nitratine and belloite. Bojarite occurs as blue fine-grained porous aggregates up to 1 mm × 3 mm × 5 mm combined typically in interrupted earthy crusts. The mineral is brittle. The Mohs hardness is 2. Dcalc = 2.057 g cm–3. The IR and Raman spectra show the presence of the 1,2,4-triazolate anion and H2O molecules. Bojarite is optically isotropic and n = 1.635(2) (λ = 589 nm). The chemical composition (electron-microprobe data for Na, Mg, Fe, Cu and Cl; H, C and N contents measured by gas chromatography on products of ignition at 1200°C; wt.%) is: Na 0.22, Mg 0.74, Fe 0.99, Cu 29.73, Cl 13.62, N 20.4, C 11.6, H 3.3, O (calculated by stoichiometry) 19.93, total 100.53.
The empirical formula is (Cu2.68Mg0.17Fe0.10Na0.05)Σ3(N3C2H2)2.755[(OH)][Cl2.19(H2O)3.77(OH)0.04]Σ6⋅2.3H2O. The idealised formula is Cu3(N3C2H2)3(OH)Cl2⋅6H2O. The crystal structure of bojarite was refined based on powder X-ray diffraction data, using the Rietveld method. The final agreement factors are: Rp = 0.0225, Rwp = 0.0310 and Robs = 0.0417. The new mineral is cubic, space group Fd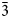 $\bar{3}$c; a = 24.8047(5) Å, V = 15,261.6(5) Å3 and Z = 32. The strongest reflections of the powder X-ray diffraction pattern [d, Å (I,%)(hkl)] are: 8.83 (31)(220), 7.19 (100)(222), 6.23 (35)(400), 5.077 (28)(422), 4.194 (28)(531), 3.584 (23)(444), 2.865 (28)(660, 751) and 2.723 (22)(753, 842).
$\bar{3}$c; a = 24.8047(5) Å, V = 15,261.6(5) Å3 and Z = 32. The strongest reflections of the powder X-ray diffraction pattern [d, Å (I,%)(hkl)] are: 8.83 (31)(220), 7.19 (100)(222), 6.23 (35)(400), 5.077 (28)(422), 4.194 (28)(531), 3.584 (23)(444), 2.865 (28)(660, 751) and 2.723 (22)(753, 842).
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2020. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Peter Leverett
References
- 4
- Cited by


