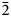Introduction
Several elements, having economic value or environmental concern, are hosted in sulfosalts, a group of complex minerals typically occurring in hydrothermal settings. Our ongoing effort in the last two decades (Bindi and Biagioni, Reference Bindi and Biagioni2018, and references therein) has been the study of these minerals from a structural point of view to try to: (1) elucidate the role played by minor components, which could give interesting insights into the physico-chemical conditions of the crystallisation environments, and (2) allow the potential description of unpredictable structures, unexpected crystallographic features, and new mineral species. Such a body of knowledge has been critical for assessing both the technological potentialities of sulfosalts as well as their geological significance.
In the course of this research project (i.e. Biagioni et al., Reference Biagioni, Bindi, Nestola, Cannon, Roth and Raber2016; Bindi et al., Reference Bindi, Nestola, Guastoni, Peruzzo, Ecker and Carampin2012a,Reference Bindi, Nestola, Guastoni, Zorzi, Peruzzo and Raberb, Reference Bindi, Biagioni, Raber, Roth and Nestola2015a,Reference Bindi, Nestola, Graeser, Tropper and Raberb), we examined a sample from the Hemlo gold deposit, Marathon, Ontario, Canada (Harris, Reference Harris1989), belonging to the mineralogical collections of the Museo di Storia Naturale of the University of Florence. The sample consists of tiny criddleite grains associated closely with aurostibite, stibarsen and native gold in a calcite matrix. Among the stibarsen fragments, a small unique grain that turned out to be biagioniite, Tl2SbS2, was found.
Biagioniite was approved as a new mineral by the International Mineralogical Association – Commission on New Minerals, Nomenclature and Classification (IMA2019-120, Bindi and Moëlo, Reference Bindi and Moëlo2020). The mineral name honours Cristian Biagioni (b. 1981), Associate Professor of Mineralogy at the Department of Earth Sciences of the University of Pisa, Italy. He is the current Italian Member of the IMA-Commission of New Minerals, Nomenclature and Classification and he is co-author of the description of more than 50 new mineral species. In addition, he contributed to the discovery of the Tl-rich nature of pyrite ores from the Apuan Alps (northern Tuscany, Italy), promoting a new scientific investigations of these ore deposits both from a mineralogical and environmental point of view (e.g. Biagioni et al., Reference Biagioni, D'Orazio, Vezzoni, Dini and Orlandi2013, Reference Biagioni, D'Orazio, Lepore, d'Acapito and Vezzoni2017). The holotype material is deposited in the mineralogical collection of the Museo di Storia Naturale of the University of Florence, Italy, under catalogue number 46582/G.
Here we report the description of the new mineral biagioniite, together with the determination of its crystal structure.
Material studied
The Museum sample containing biagioniite comes from the Hemlo gold deposit, which is located near the northeast shore of Lake Superior, 35 km east of Marathon, Ontario (Harris, Reference Harris1989; Tomkins et al., Reference Tomkins, Pattison and Zaleski2004). The deposit, discovered in 1982, is of Archean age and occurs at the contact of felsic metavolcanics and pelitic metasediments. It consists of several mineralised zones, of which the main zone extends for a length of 2900 m, for a distance of 2500 m down-dip and ranges in thickness from 3 to 45 m. The ore minerals were formed from hydrothermal fluids that may in part be related to the shear zone.
Native gold is the principal gold mineral in the deposit (Harris, Reference Harris1989). The gold ore is substantially enriched in Mo, V, As, Sb, Hg, Tl and Ba and contains a diverse assemblage of minerals. Interestingly, there is a close spatial association between the mercury and thallium minerals. In this regard, the realgar–cinnabar-rich quartz veins that occur within the central portion of the deposit are common hosts for the thallium minerals (Harris, Reference Harris1989). Routhierite is the most common thallium mineral, parapierrotite is less abundant, while vaughanite and criddleite are rare.
Physical and optical properties
Biagioniite occurs as very rare crystals grown on a calcite matrix (Fig. 1). The mineral exhibits a subhedral to anhedral grain morphology, and shows no inclusions of, or intergrowths with, other minerals. The maximum grain size of biagioniite is ~65 μm. It is black in colour and shows a black streak. The mineral is opaque in transmitted light and exhibits a metallic lustre. No cleavage is observed, and the fracture is uneven. The calculated density (for Z = 8) for the empirical formula (see below) is 6.192 g/cm3. Unfortunately, the density could not be measured here because of the small grain size. The Mohs hardness, estimated with respect to the surrounding calcite, is ~3.

Fig. 1. Incident-light image of biagioniite associated with stibarsen on a calcite matrix. Museo di Storia Naturale of the University of Florence, catalogue number 46582/G.
In plane-polarised incident light, biagioniite is creamy in colour, moderately bireflectant and not pleochroic. Between crossed polars, biagioniite is weakly anisotropic with blueish to light-blue rotation tints. Internal reflections are absent and there is no optical evidence of growth zonation.
Reflectance measurements were performed in air by means of a MPM-200 Zeiss microphotometer equipped with a MSP-20 system processor on a Zeiss Axioplan ore microscope. The filament temperature was ~3350 K. An interference filter was adjusted, in turn, to select four wavelengths for measurement (471.1, 548.3, 586.6 and 652.3 nm). Readings were taken for specimen and standard (SiC) maintained under the same focus conditions. The diameter of the circular measuring area was 0.04 mm. Reflectance percentages for R min and R max are: 35.9 and 37.5 (471.1 nm); 34.7 and 36.2 (548.3 nm); 33.8 and 35.3 (586.6 nm); and 31.5 and 33.7 (652.3 nm), respectively.
Chemical composition
A preliminary chemical analysis using energy-dispersive spectrometry performed on the crystal fragment used for the structural study did not indicate the presence of elements (Z > 9) other than Tl, Sb, S and minor Ag. Analyses were carried out using a JEOL 8200 microprobe (wavelength dispersive spectrometry mode, 25 kV, 20 nA, 1 μm beam size, counting times 20 s for peak and 10 s for background). The following lines were used: AgLα, TlMα, SbLβ and SKα. The standards employed were: synthetic TlI (Tl), pure element (Ag), synthetic Sb2Te3 (Sb) and pyrite (S). The crystal fragment was found to be homogeneous within analytical error. The average chemical compositions (four analyses on different spots) together with wt.% ranges of elements are reported in Table 1. On the basis of 5 atoms, the empirical formula of biagioniite is (Tl1.87Ag0.19)Σ2.06Sb0.97S1.97. The simplified ideal formula is (Tl,Ag)2SbS2, and the ideal formula is Tl2SbS2 (Z = 8), which requires Tl 68.74, Sb 20.48, S 10.78, total 100 wt.%.
Table 1. Electron microprobe analysis results (four analytical spots, wt.% of elements) for biagioniite.

X-ray crystallography and crystal-structure determination
The same crystal fragment (40 × 50 × 65 μm) used to obtain the chemical data was selected for X-ray single-crystal diffraction. Data were collected using a Bruker D8 Venture diffractometer equipped with an Photon II CCD detector, with graphite-monochromatised MoKα radiation (λ = 0.71073 Å). Biagioniite was found to be monoclinic, with a = 11.0895(9), b = 14.3124(11), c = 7.9352(6) Å, β = 96.230(8)°, V = 1252.02(17) Å3 and Z = 8. The analysis of the systematic absences (h0l: l = 2n and 00l: l = 2n) led to the choice of the space groups Pc and P2/c. Although the statistical tests on the distribution of |E| values (|E 2–1| = 0.812) indicated the absence of an inversion centre, suggesting the choice of the space group Pc, the structure was preliminarily solved in the P2/c space group. A residual R 1 = 0.18 value was achieved quickly. However, the preliminary structural model obtained indicated a large atomic disorder. The structure model was subsequently optimised, and an ordered model was sought, but no improvement in R could be achieved. At this point, a thorough analysis of the structure (essentially based upon the observation of the very large atomic displacement parameters for particular atoms) suggested that some symmetry element of the P2/c space group should be removed. The reflection and atomic position data sets were then adapted to the Pc space group (showing the same reflections conditions) and the structure refined. After several cycles, an ordered solution with full site occupancies was finally determined by carefully removing atoms with low site occupancies and/or non-realistic distances with neighbouring atoms and adding significant positions found in the difference-Fourier syntheses. The structure could be smoothly refined in Pc without any damping factor or restrictions by the program SHELXL (Sheldrick, Reference Sheldrick2008). The occupancy of all the sites was left free to vary (Tl vs. □; Sb vs. □; S vs. □, where □ = a vacancy) but all the positions were found to be fully occupied. Neutral scattering curves for Tl, As and S were taken from the International Tables for X-ray Crystallography (Wilson, Reference Wilson1992). At the last stage, with anisotropic atomic displacement parameters for all the atoms and no constraints, the residual value settled at R 1 = 0.0243 for 2655 observed reflections [2σ(I) level] and 182 parameters and at R 1 = 0.0315 for all 4520 independent reflections.
Note that the acentric structural model we obtained does not show high values in the correlation matrix between pairs of atoms which are equivalent in the centrosymmetric space group P2/c. To test whether the acentric model is to be preferred to the centric one we also tested the presence of twinning by inversion in the non-centrosymmetric structure refinement. Indeed, as is well known, a centrosymmetric structure that is refined as non-centrosymmetric will show a twin scale factor, equivalent to the Flack parameter in the case of inversion twinning (Flack et al., Reference Flack, Bernardinelli, Clemente, Lindenc and Spek2006; Müller et al., Reference Müller, Herbst-Irmer, Spek, Schneider and Sawaya2006), that refines to 50% within analytical uncertainty. We found the racemic twin-component scale factor refined to 0.09(2), consistent with a highly asymmetrical distribution of the enantiomorphic components and indicating the acentric model as the right choice.
Experimental details and R indices are given in Table 2. Fractional atomic coordinates and atomic displacement parameters are reported in Table 3. Bond distances are given in Table 4. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 2. Crystallographic data and experimental details for the selected biagioniite crystal.

Table 3. Atoms, fractional atom coordinates (Å), and atomic displacement parameters (Å2) for biagioniite.

Table 4. Selected bond distances (Å) for biagioniite.

Powder X-ray diffraction data (Table 5) were collected with an Oxford Diffraction Excalibur PX Ultra diffractometer fitted with a 165 mm diagonal Onyx CCD detector and using copper radiation (CuKα, λ = 1.54138 Å). The least squares refinement gave the following values: a = 11.0999(9), b = 14.273(1), c = 7.9323(7) Å, β = 96.29(1)° and V = 1249.1(1) Å3.
Table 5. Observed and calculated* powder X-ray diffraction data (d in Å) for biagioniite

*Calculated diffraction pattern obtained with the atom coordinates and occupancies reported in Table 3 (only reflections with I rel ≥ 8 are listed).
Description of the structure and discussion
The structure of biagioniite projected down [001] is reported in Fig. 2. It is isostructural with dervillite, Ag2AsS2 (Bindi et al., Reference Bindi, Nestola, De Battisti and Guastoni2013). An enlarged projection of the structure (Fig. 3) allows one to distinguish Tl4(Sb2)2S6 ribbons parallel to [001], stacked along [010], to form undulated layers along [100]. These layers are separated by a one-atom-thick Tl4S2 layer. It is noteworthy, there are two Sb–Sb pairs, Sb1–Sb3 (2.843 Å) and Sb2–Sb4 (2.830 Å).

Fig. 2. The crystal structure of biagioniite down [001]: unit-cell content, atoms labels and Sb–Sb bond lengths (Å). Tl, Sb and S atoms are given as purple, green and yellow circles, respectively.

Fig. 3. Biagioniite: 2a × 2b cell. Tl4(Sb2)2S6 ribbons parallel to [001], stacked along [010], form undulated layers along [100]. These layers are separated by a one-atom-thick layer of Tl4S2.
The coordination of these pairs is represented in Fig. 4. Coordination of each pair with four S atoms corresponds to a hemi-octahedron cut along a symmetry plane (through two opposite edges of the cube – Fig. 5). Two neighbouring hemi-octahedra brought closer allow a single octahedron to be rebuilt.

Fig. 4. Coordination of the Sb pairs with S atoms.

Fig. 5. Coordination of each Sb pair (down, reduced to a central blue atom) with four S atoms corresponding to a hemi-octahedron cut along a symmetry plane (through two opposite edges of the cube). Two neighbouring hemi-octahedra brought closer allow the rebuilding of the octahedron (above).
In the Tl4(Sb2)2S6 ribbon layer, Tl1 and Tl3 exhibit a tetrahedral coordination with S, whereas the coordination is triangular for Tl4 and Tl5. Nevertheless, the coordination environment for Tl4 and Tl5 is completed by additional short Tl–Sb bonds (Tl5–Sb1 = 3.067 Å and Tl4–Sb4 = 3.175 Å).
The coordination of Tl atoms of the Tl4S2 layer is shown in Fig. 6. There is a central zig-zag row of tetrahedral Tl atoms, flanked by two stripes of triangular Tl, and then two stripes of linear Tl. Contrary to the Tl4(Sb2)2S6 ribbon-layer (see below), here there is linear Tl on one mirror position, and tetrahedral Tl along the second mirror position. It is worth noting the linear coordination of Tl7. To the best of our knowledge, biagioniite seems to be the first example of a natural chalcogenide showing Tl in linear coordination. For a review of Tl chalcogenides see Makovicky (Reference Makovicky2018).

Fig. 6. Coordination of Tl atoms in the Tl4S2 layer in the structure of biagioniite. Tl2: tetrahedral; Tl6 and Tl8: triangular; Tl7: linear.
In Fig. 7, the Sb2 pairs have been replaced by a single atom (G) at their gravity centre, and an anionic vacancy (□) has been added. One Tl4(Sb2)2S6 ribbon (now Tl4G2S6□2) has been selected. Projection of this ribbon (Fig. 8) shows that it is a distorted derivative of the PbS structure. The Pb6S8 ideal ribbon (Fig. 9) is parallel to [310], two-atoms-thick, and three-octahedra large. The junction between two ribbons along [010] (mirror in the structure, with a shift along c, to preclude S–S short bond in the interface) corresponds to (131) of PbS. The two sides of such a junction (the two different mirror positions) have the same topology.

Fig. 7. Sb2 pairs have been replaced by a single atom (G, green) at their gravity centre, and an anionic vacancy (□, red) has been added. One Tl4(Sb2)2S6 ribbon (now Tl4G2S6□2) has been selected.

Fig. 8. One Tl4G2S6□2 ribbon in the crystal structure of biagioniite can be described as a distorted derivative of PbS structure.

Fig. 9. A Pb6S8 ideal ribbon parallel to [310], two-atoms-thick, and three-octahedra large. The junction between two ribbons in the structure of biagioniite along [010] corresponds to (131) of PbS.
Bond-valence calculations (Table 6) have been computed on the basis of the following bond parameters: R Sb,S = 2.45 (Brese and O'Keeffe, Reference Brese and O'Keeffe1991), R Tl,S = 2.55 (Biagioni et al., Reference Biagioni, Bonaccorsi, Moëlo, Orlandi, Bindi, D'Orazio and Vezzoni2014) and R Sb,Sb = 2.82 (O'Keeffe and Brese, Reference O'Keeffe and Brese1992). Tl atoms are overbonded (bond valence from 1.11 up to 1.36 valence units, vu), but we have not considered in the computation the presence of minor Ag disordered at the Tl positions, although it would be very minor. Sb–Sb bond lengths (2.830 and 2.843 Å) agree well with R Sb,Sb corresponding to one vu; nevertheless, Sb atoms are underbonded varying from 2.31 to 2.57 vu. This feature has also been observed in sartorite (Berlepsch et al. Reference Berlepsch, Armbruster, Makovicky and Topa2003) and in minerals such as dadsonite (Makovicky et al., Reference Makovicky, Topa and Mumme2006), and was discussed in detail by Mills et al. (Reference Mills, Christy, Chen and Raudsepp2009). The overall bond-valence sum of the eight S sites is 15.95, very close to the theoretical value (16 vu). These results may be due to uncertainty in the positions of some S atoms: a small shift closer to Sb atoms would reduce Sb underbonding together with Tl overbonding, without significant change of the S bond-valence sum.
Table 6. Bond-valence sums (vu) for biagioniite.

The presence of dimeric [Sb2S4]4– ions with a central Sb–Sb bond in biagioniite means the formula could be written as [Tl+1]4[Sb2]4+[S2–]4. However, it is difficult to analyse such polycationic compounds in strict bond-valence terms, as the electronegativity of such elements lies between that of common cations and common anions. The weak Tl–Sb bonds in biagioniite are good examples of the ‘anionic’ behaviour, which could be explained through dative donation of the Sb lone pair to the closed-shell d 10 Tl cations. On the contrary, in dervillite, short Ag–As bonds are lacking (Bindi et al., Reference Bindi, Nestola, De Battisti and Guastoni2013).
Tl2SbS2 has never been described either in Nature or as synthetic compound. Actually, the experimental TlSbS2–Tl section includes three ternary compounds, namely Tl2SbS2, Tl4SbS2 and Tl5SbS2. The former two compounds melt congruently at 613 and 683 K, whereas the latter one decomposes by peritectic reaction at 663 K (Jafarov et al., Reference Jafarov, Ismayilova, Aliev, Imamaliyeva, Yusibov and Babanly2016). But, in fact, the Tl2SbS2 compound has been found to be Tl3SbS3 + Sb – a two-phase mixture instead of a single, independent chemical compound. Such a S-deficient formula for biagioniite indicates its formation at Hemlo at low ![]() $f_{{\rm S}_ 2}$, in accordance to its association with stibarsen, SbAs, aurostibite, AuSb2, and criddleite, TlAg2Au3Sb10S10 (also S-deficient).
$f_{{\rm S}_ 2}$, in accordance to its association with stibarsen, SbAs, aurostibite, AuSb2, and criddleite, TlAg2Au3Sb10S10 (also S-deficient).
Acknowledgements
The manuscript benefited from the reviews of Federica Zaccarini, Stuart Mills, and an anonymous reviewer. The research was funded by MIUR-PRIN2017, project “TEOREM deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32 (PI: Luca Bindi).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2020.27