Article contents
Belousovite, KZn(SO4)Cl, a new sulfate mineral from the Tolbachik volcano with apophyllite sheet-topology.
Published online by Cambridge University Press: 15 May 2018
Abstract
Belousovite, ideally KZn(SO4)Cl, was found in a Yadovitaya fumarole of the Second scoria cone of the North Breach of the Great Tolbachik Fissure Eruption (1975–1976), Tolbachik volcano, Kamchatka Peninsula, Russia. Belousovite occurs as irregularly-shaped grains and in the form of microcrystalline masses associated with kamchatkite, langbeinite, euchlorine, anglesite and zincite. Belousovite is monoclinic, P21/c, a = 6.8904(5), b = 9.6115(7), c = 8.2144(6) Å, β = 96.582(2), V = 540.43(7) Å3 and Z = 4 (from single-crystal diffraction data). The eight strongest lines of the powder X-ray diffraction pattern are [dmeas Å(I)(hkl)]: 6.8451(100)(100), (3.6401)(71)(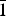 $\bar{1}$21), (3.1592)(84)(1
$\bar{1}$21), (3.1592)(84)(1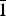 $\bar{1}$2), (3.1218)(41)(
$\bar{1}$2), (3.1218)(41)(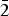 $\bar{2}$11), (3.1140)(52)(022), (2.9812)(41)(031), (2.9121)(44)(130) and (2.0483)(19)(
$\bar{2}$11), (3.1140)(52)(022), (2.9812)(41)(031), (2.9121)(44)(130) and (2.0483)(19)(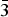 $\bar{3}$12). The chemical composition determined by the electron-microprobe analysis is (wt.%): K2O 19.55, Rb2O 0.58, ZnO 34.85, SO3 34.65, Cl 14.77, –O = Cl2 3.34, total 101.06. The empirical formula based on O + Cl = 5 apfu is K0.97Rb0.01Zn1.00S1.01O4.03Cl0.97. The simplified formula is KZn(SO4)Cl. The crystal structure was solved by direct methods and refined to R1 = 0.029 on the basis of 1965 independent observed reflections. The structure of belousovite consists of infinite [ZnSO4Cl]– layers and K+ ions. [ZnSO4Cl]– layers are formed by corner sharing mixed-ligand ZnO3Cl tetrahedra and SO4 tetrahedra. The topology of [ZnSO4Cl]– layers in belousovite is identical to [Si4O10]4– layers in the minerals of the apophyllite group. A review of mixed-ligand ZnOmCln coordination polyhedra in minerals and inorganic compounds is given.
$\bar{3}$12). The chemical composition determined by the electron-microprobe analysis is (wt.%): K2O 19.55, Rb2O 0.58, ZnO 34.85, SO3 34.65, Cl 14.77, –O = Cl2 3.34, total 101.06. The empirical formula based on O + Cl = 5 apfu is K0.97Rb0.01Zn1.00S1.01O4.03Cl0.97. The simplified formula is KZn(SO4)Cl. The crystal structure was solved by direct methods and refined to R1 = 0.029 on the basis of 1965 independent observed reflections. The structure of belousovite consists of infinite [ZnSO4Cl]– layers and K+ ions. [ZnSO4Cl]– layers are formed by corner sharing mixed-ligand ZnO3Cl tetrahedra and SO4 tetrahedra. The topology of [ZnSO4Cl]– layers in belousovite is identical to [Si4O10]4– layers in the minerals of the apophyllite group. A review of mixed-ligand ZnOmCln coordination polyhedra in minerals and inorganic compounds is given.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: Ian Graham
References
- 10
- Cited by


