Published online by Cambridge University Press: 05 July 2018
Aspidolite, the Na analogue of phlogopite, ideally NaMg3AlSi3O10(OH)2, occurring in hornfels from a contact aureole in Kasuga-mura, central Japan, has been approved as a mica species by the Commission on New Minerals and Mineral Names of the International Mineralogical Association. Aspidolite is interleaved with and surrounded by phlogopite. Based on its mode of occurrence, phlogopite is classified into two types; (1) phlogopite interleaved with aspidolite (= interleaved phlogopite) and (2) phlogopite rim. The aspidolite-phlogopite assemblage is associated with amphibole (pargasite-magnesiosadanagaite), titanite, calcite, scapolite, apatite, pyrrhotite and chalcopyrite. A representative chemical formula of aspidolite is (Na0.90K0.10)∑1.00(Mg2.27Al0.41Fe0.232+Ti0.05)∑2.96 (Al1.44Si2.56)∑4.00O10(OH1.97F0.03)∑2.00. Aspidolite has almost fully occupied the interlayer site; its Na/(Na+K) ratio ranges from 0.67 to 0.95. It has more tetrahedral Al (1.38—1.48 a.p.f.u. for O = 11) than the ideal aspidolite end-member showing progression of tschermakite-type substitution. The alternation of aspidolite and phlogopite parallel to the (001) plane may indicate a miscibility gap between these two phases. The phlogopite rim is interpreted as a later product, probably formed metasomatically. Aspidolite is optically biaxial negative with elongation positive and Z ‖ cleavage. Two polytypes (1M and 1A) of aspidolite were identified in X-ray powder diffraction patterns. Aspidolite-1M is monoclinic, space group C2/m, with refined unit-cell parameters a = 5.291(8), b = 9.16(2), c = 10.12(2) Å, β = 105.1(1)°, V = 473(1) Å3, Z = 2. Aspidolite-1A is triclinic, space group C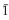 , with a = 5.289(6), b = 9.16(1), c = 9.892(9) Å, α = 94.45(9), β = 97.74(9), γ = 90.0(1)°, V = 473.4(9) Å3, Z = 2.
, with a = 5.289(6), b = 9.16(1), c = 9.892(9) Å, α = 94.45(9), β = 97.74(9), γ = 90.0(1)°, V = 473.4(9) Å3, Z = 2.