Published online by Cambridge University Press: 05 July 2018
Arisite-(La), ideally NaLa2(CO3)2[F2x(CO3)1–x]F, is a new layered REE-fluorcarbonate mineral from miarolitic cavities within the Aris phonolite, Namibia (IMA no. 2009-019). It occurs as distinct chemical zones mixed with its Ce-analogue, arisite-(Ce). Crystals are vitreous, transparent beige, beige-yellow, light lemon-yellow to pinkish, and occur as tabular prisms up to 1.5 mm. Arisite-(La) is brittle, has conchoidal fracture, poor cleavage perpendicular to (001), a Mohs hardness of ~3–3½, is not fluorescent in either long- or shortwave UV radiation, dissolves slowly in dilute HCl at room temperature and sinks in methylene iodide, Dcalc. = 4.072 g cm–3. Arisite-(La) is uniaxial negative, has sharp extinction, with both ω and ε exhibiting a range of values within each grain: ω = 1.696–1.717(4) and ε = 1.594–1.611(3), a result of chemical zoning attributed to both Ce ⇌ La and Na ⇌ Ca substitutions. The crystal structure of both arisite-(Ce) and arisite-(La) were solved by direct methods and refined to R = 1.66%, wR2 = 4.31% (Ce) and R = 2.09%, wR2 = 5.26% (La), respectively. Arisite is hexagonal, P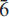 m2, Z = 1, with unit-cell parameters of a = 5.1109(2) Å, c = 8.6713(4) Å, V = 196.16(6) Å3 for arisite-(Ce), and a = 5.1131(7) Å, c = 8.6759(17) Å, V = 196.43(5) Å3 for arisite-(La). Arisite-(Ce) and arisite-(La) are members of the layered, flat-lying REE-fluorcarbonate group which have crystal structures characterized by separate layers of triangular planar
m2, Z = 1, with unit-cell parameters of a = 5.1109(2) Å, c = 8.6713(4) Å, V = 196.16(6) Å3 for arisite-(Ce), and a = 5.1131(7) Å, c = 8.6759(17) Å, V = 196.43(5) Å3 for arisite-(La). Arisite-(Ce) and arisite-(La) are members of the layered, flat-lying REE-fluorcarbonate group which have crystal structures characterized by separate layers of triangular planar 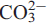 groups that parallel the overall layering of the structure, F, REE and alkali or alkaline-earthelements. Overall, the arisite structure can be defined by three distinct layers which parallel (001): (1) ∞[REE(CO3)2F] slabs, (2) sheets of Naϕ9 polyhedra, and (3) ∞[2F/CO3]2–. Based on its (M+F)/C ratio, arisite can further be described as having a dense, flat-lying fluorcarbonate structure, a classification which includes the structurally related mineral species cordylite, kukharenkoite, cebaite, lukechangite, huanghoite, and one incompletely characterized synthetic phase, NaY2(CO3)3F.
groups that parallel the overall layering of the structure, F, REE and alkali or alkaline-earthelements. Overall, the arisite structure can be defined by three distinct layers which parallel (001): (1) ∞[REE(CO3)2F] slabs, (2) sheets of Naϕ9 polyhedra, and (3) ∞[2F/CO3]2–. Based on its (M+F)/C ratio, arisite can further be described as having a dense, flat-lying fluorcarbonate structure, a classification which includes the structurally related mineral species cordylite, kukharenkoite, cebaite, lukechangite, huanghoite, and one incompletely characterized synthetic phase, NaY2(CO3)3F.