Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Morizet, Y.
Brooker, R.A.
and
Kohn, S.C.
2002.
CO 2 in haplo-phonolite melt: solubility, speciation and carbonate complexation.
Geochimica et Cosmochimica Acta,
Vol. 66,
Issue. 10,
p.
1809.
Nowak, Marcus
Schreen, Dominik
and
Spickenbom, Kai
2004.
Argon and CO2 on the race track in silicate melts: A tool for the development of a CO2 speciation and diffusion model.
Geochimica et Cosmochimica Acta,
Vol. 68,
Issue. 24,
p.
5127.
Behrens, Harald
Ohlhorst, Susanne
Holtz, Francois
and
Champenois, Michel
2004.
CO2 solubility in dacitic melts equilibrated with H2O-CO2 fluids: Implications for modeling the solubility of CO2 in silicic melts.
Geochimica et Cosmochimica Acta,
Vol. 68,
Issue. 22,
p.
4687.
Botcharnikov, R.E.
Behrens, H.
and
Holtz, F.
2006.
Solubility and speciation of C–O–H fluids in andesitic melt at T=1100–1300°C and P=200 and 500MPa.
Chemical Geology,
Vol. 229,
Issue. 1-3,
p.
125.
Morizet, Y.
Paris, M.
Gaillard, F.
and
Scaillet, B.
2009.
Raman quantification factor calibration for CO–CO2 gas mixture in synthetic fluid inclusions: Application to oxygen fugacity calculation in magmatic systems.
Chemical Geology,
Vol. 264,
Issue. 1-4,
p.
58.
Behrens, Harald
2010.
Ar, CO2 and H2O diffusion in silica glasses at 2 kbar pressure.
Chemical Geology,
Vol. 272,
Issue. 1-4,
p.
40.
Morizet, Y.
Paris, M.
Gaillard, F.
and
Scaillet, B.
2010.
C–O–H fluid solubility in haplobasalt under reducing conditions: An experimental study.
Chemical Geology,
Vol. 279,
Issue. 1-2,
p.
1.
Spickenbom, K.
Sierralta, M.
and
Nowak, M.
2010.
Carbon dioxide and argon diffusion in silicate melts: Insights into the CO2 speciation in magmas.
Geochimica et Cosmochimica Acta,
Vol. 74,
Issue. 22,
p.
6541.
Balta, J. Brian
Asimow, Paul D.
and
Mosenfelder, Jed L.
2011.
Hydrous, Low-carbon Melting of Garnet Peridotite.
Journal of Petrology,
Vol. 52,
Issue. 11,
p.
2079.
Guillot, Bertrand
and
Sator, Nicolas
2011.
Carbon dioxide in silicate melts: A molecular dynamics simulation study.
Geochimica et Cosmochimica Acta,
Vol. 75,
Issue. 7,
p.
1829.
Mysen, Bjorn O.
2012.
Silicate-COH melt and fluid structure, their physicochemical properties, and partitioning of nominally refractory oxides between melts and fluids.
Lithos,
Vol. 148,
Issue. ,
p.
228.
Klimm, Kevin
Kohn, Simon C.
and
Botcharnikov, Roman E.
2012.
The dissolution mechanism of sulphur in hydrous silicate melts. II: Solubility and speciation of sulphur in hydrous silicate melts as a function of fO2.
Chemical Geology,
Vol. 322-323,
Issue. ,
p.
250.
Keppler, Hans
2013.
Physics and Chemistry of the Deep Earth.
p.
1.
Morizet, Yann
Paris, Michael
Di Carlo, Ida
and
Scaillet, Bruno
2013.
Effect of sulphur on the structure of silicate melts under oxidizing conditions.
Chemical Geology,
Vol. 358,
Issue. ,
p.
131.
Shishkina, Tatiana A.
Botcharnikov, Roman E.
Holtz, Francois
Almeev, Renat R.
Jazwa, Aleksandra M.
and
Jakubiak, Artur A.
2014.
Compositional and pressure effects on the solubility of H2O and CO2 in mafic melts.
Chemical Geology,
Vol. 388,
Issue. ,
p.
112.
Duncan, Megan S.
and
Dasgupta, Rajdeep
2014.
CO2 solubility and speciation in rhyolitic sediment partial melts at 1.5–3.0GPa – Implications for carbon flux in subduction zones.
Geochimica et Cosmochimica Acta,
Vol. 124,
Issue. ,
p.
328.
Di Genova, D.
Romano, C.
Alletti, M.
Misiti, V.
and
Scarlato, P.
2014.
The effect of CO2 and H2O on Etna and Fondo Riccio (Phlegrean Fields) liquid viscosity, glass transition temperature and heat capacity.
Chemical Geology,
Vol. 377,
Issue. ,
p.
72.
Konschak, Alexander
and
Keppler, Hans
2014.
The speciation of carbon dioxide in silicate melts.
Contributions to Mineralogy and Petrology,
Vol. 167,
Issue. 5,
Vuilleumier, Rodolphe
Seitsonen, Ari P.
Sator, Nicolas
and
Guillot, Bertrand
2015.
Carbon dioxide in silicate melts at upper mantle conditions: Insights from atomistic simulations.
Chemical Geology,
Vol. 418,
Issue. ,
p.
77.
Duncan, Megan S.
and
Dasgupta, Rajdeep
2015.
Pressure and temperature dependence of CO2 solubility in hydrous rhyolitic melt: implications for carbon transfer to mantle source of volcanic arcs via partial melt of subducting crustal lithologies.
Contributions to Mineralogy and Petrology,
Vol. 169,
Issue. 6,
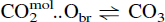 1
1