Published online by Cambridge University Press: 12 April 2024
The new eudialyte-group mineral amableite-(Ce), ideally Na15[(Ce1.5Na1.5)Mn3]Mn2Zr3□Si[Si24O69(OH)3](OH)2⋅H2O, was discovered in a peralkaline pegmatite at Saint-Amable Sill, Montérégie, Québec, Canada. The associated minerals are albite, microcline, aegirine, serandite, natrolite, yofortierite, and an unidentified titanosilicate forming minute grains. Amableite-(Ce) occurs as yellow equant or thick tabular crystals up to 2 mm across. The observed crystal forms are {0001}; the subordinate forms are {11$\bar{2}$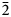 0}, {10$\bar{1}$
0}, {10$\bar{1}$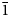 1}, and {10$\bar{1}$
1}, and {10$\bar{1}$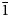 0}. Amableite-(Ce) is brittle, with a Mohs hardness of 5. D(meas) = 2.89(1), D(calc) = 2.899 g⋅cm–3. Amableite-(Ce) is optically anomalously biaxial (+) with α ≈ β = 1.603(2) and γ = 1.608(2). The chemical composition is (wt.%, electron microprobe, H2O measured by means of a modified Penfield method): Na2O 14.20, K2O 0.41, CaO 1.89, MnO 8.25, Fe2O3 2.40, La2O3 3.10, Ce2O3 4.19, Pr2O3 0.16, Nd2O3 0.59, SiO2 49.41, ZrO2 11.17, HfO2 0.24, TiO2 0.68, Nb2О5 1.54, Cl 0.26, H2O 1.70, –O≡Cl –0.06, total 100.13. The crystal structure was determined using single-crystal X-ray diffraction data and refined to R1 = 0.0423. Amableite-(Ce) is trigonal, space group R3, with a = 14.1340(3) Å, c = 30.3780(11) Å and V = 5255.6(3) Å3. The crystal-chemical formula is (Na12.93K0.27Ce0.06)Σ13.26[(Mn2.49Ce0.30Ca0.21)Σ3.00(Ce1.14Na1.04Ca0.82)Σ3.00](Mn1.05Fe0.90□1.05)Σ3.00(Zr2.85Ti0.12Hf0.03)Σ3.00(□0.40Nb0.36Si0.24)Σ1.00(Si0.88□0.12)Σ1.00[Si24(O70.44(OH)1.56)Σ72.00][(OH)2.20(H2O)1.27]Σ3.47Cl0.22 (Z = 3). Infrared and Raman spectra are given. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %)(hkl)] are: 11.34 (51)(101), 7.06 (76)(110), 4.312 (63)(205), 3.783 (38)(033), 3.538 (43)(027, 220), 2.963 (84)($\bar{3}$
0}. Amableite-(Ce) is brittle, with a Mohs hardness of 5. D(meas) = 2.89(1), D(calc) = 2.899 g⋅cm–3. Amableite-(Ce) is optically anomalously biaxial (+) with α ≈ β = 1.603(2) and γ = 1.608(2). The chemical composition is (wt.%, electron microprobe, H2O measured by means of a modified Penfield method): Na2O 14.20, K2O 0.41, CaO 1.89, MnO 8.25, Fe2O3 2.40, La2O3 3.10, Ce2O3 4.19, Pr2O3 0.16, Nd2O3 0.59, SiO2 49.41, ZrO2 11.17, HfO2 0.24, TiO2 0.68, Nb2О5 1.54, Cl 0.26, H2O 1.70, –O≡Cl –0.06, total 100.13. The crystal structure was determined using single-crystal X-ray diffraction data and refined to R1 = 0.0423. Amableite-(Ce) is trigonal, space group R3, with a = 14.1340(3) Å, c = 30.3780(11) Å and V = 5255.6(3) Å3. The crystal-chemical formula is (Na12.93K0.27Ce0.06)Σ13.26[(Mn2.49Ce0.30Ca0.21)Σ3.00(Ce1.14Na1.04Ca0.82)Σ3.00](Mn1.05Fe0.90□1.05)Σ3.00(Zr2.85Ti0.12Hf0.03)Σ3.00(□0.40Nb0.36Si0.24)Σ1.00(Si0.88□0.12)Σ1.00[Si24(O70.44(OH)1.56)Σ72.00][(OH)2.20(H2O)1.27]Σ3.47Cl0.22 (Z = 3). Infrared and Raman spectra are given. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %)(hkl)] are: 11.34 (51)(101), 7.06 (76)(110), 4.312 (63)(205), 3.783 (38)(033), 3.538 (43)(027, 220), 2.963 (84)($\bar{3}$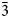 45), 2.837 (100)(404). The mineral is named after the discovery locality.
45), 2.837 (100)(404). The mineral is named after the discovery locality.
Associate Editor: Daniel Atencio