Published online by Cambridge University Press: 05 July 2018
Vysokýite, U4+[(AsO2(OH)2]4(H2O)4 (IMA 2012–067), was found growing on an altered surface of massive native As in the Geschieber vein, Jáchymov ore district, Western Bohemia, Czech Republic. The new mineral was found in association with běhounekite, štěpite, kaatialaite, arsenolite, claudetite and gypsum. It forms extremely fibrous light-green crystals up to 8 mm long. Crystals have an alabaster lustre and a greenish-white to greyish streak. Vysokýite is brittle with uneven fracture and perfect cleavage along (100) and (001); the Mohs hardness is ∼2. A density of 3.393 g/cm3 was calculated using the empirical formula and unit-cell parameters obtained from a single-crystal diffraction experiment. Vysokýite is non-fluorescent under short or long wavelength UV radiation. It is colourless under the microscope, measured refractive indices are α' = 1.617(3), γ' = 1.654(3); the estimated optical orientation is α' ∼X, γ' ∼Z. The average of five spot wavelength dispersive spectroscopy (WDS) analyses is 29.44 UO2, 1.03 SiO2, 48.95 As2O5, 0.12 SO3, 15.88 H2O (calc.), total 95.42 wt.%. The empirical formula of vysokýite (based on 20 O a.p.f.u.) is U1.00[AsO2(OH)2]3.90(SiO4)0.16 (SO4)0.01·4H2O. The As–O–H and O–H vibrations dominate in the Raman spectrum. Vysokýite is triclinic, space group P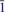 , with a = 10.749(2), b = 5.044(3), c = 19.1778(7) Å, α = 89.872(15)°, β = 121.534(15)°, γ = 76.508(15)°, and V = 852.1(6) Å3, Z = 2 and Dcalc = 3.34 g·cm–3. The strongest diffraction peaks in the X-ray powder diffraction pattern are [dobs in Å (Irel.)(hkl)]: 8.872(100)(100), 8.067(50)(002), 6.399(7)(10
, with a = 10.749(2), b = 5.044(3), c = 19.1778(7) Å, α = 89.872(15)°, β = 121.534(15)°, γ = 76.508(15)°, and V = 852.1(6) Å3, Z = 2 and Dcalc = 3.34 g·cm–3. The strongest diffraction peaks in the X-ray powder diffraction pattern are [dobs in Å (Irel.)(hkl)]: 8.872(100)(100), 8.067(50)(002), 6.399(7)(10 ), 4.773(6)(10
), 4.773(6)(10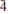 ), 3.411(10)(30
), 3.411(10)(30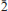 ), 3.197(18)(31
), 3.197(18)(31 ). The crystal structure of vysokýite was solved from single-crystal X-ray diffraction data by the charge-flipping method and refined to R1 = 0.0595 based on 2718 unique observed reflection, and to wR2 = 0.1160 for all 4173 unique reflections. The structure of vysokýite consists of UO8 square antiprisms sharing all of their vertices with 8 As-tetrahedra to form infinite chains parallel to [010]. These chains are linked by hydrogen bonds involving terminal (OH) groups of the double-protonated As-tetrahedra and molecules of H2O located between the chains. The new mineral is named in honour of Arnošt Vysoký (1823–1872), the former chief of the Jáchymov mines and smelters, chemist and metallurgist.
). The crystal structure of vysokýite was solved from single-crystal X-ray diffraction data by the charge-flipping method and refined to R1 = 0.0595 based on 2718 unique observed reflection, and to wR2 = 0.1160 for all 4173 unique reflections. The structure of vysokýite consists of UO8 square antiprisms sharing all of their vertices with 8 As-tetrahedra to form infinite chains parallel to [010]. These chains are linked by hydrogen bonds involving terminal (OH) groups of the double-protonated As-tetrahedra and molecules of H2O located between the chains. The new mineral is named in honour of Arnošt Vysoký (1823–1872), the former chief of the Jáchymov mines and smelters, chemist and metallurgist.