Published online by Cambridge University Press: 09 November 2018
Voudourisite, Cd(SO4)·H2O, and lazaridisite, Cd3(SO4)3·8H2O, are two new minerals from the Esperanza Mine, Kaminiza Area, Lavrion Mining District, Greece. This small ancient mine is also the type locality of niedermayrite and katerinopoulosite. Further associated minerals are sphalerite, galena, edwardsite, chalcanthite, gypsum and greenockite. Both secondary minerals form tiny clusters or crusts and are among the latest to form in this paragenetic sequence. They are colourless or white, transparent to translucent, with a white streak and vitreous lustre. No fluorescence is observed. The minerals exhibit conchoidal fracture, no cleavage or preferred parting. The Mohs hardness is ~3 and both have brittle tenacity. Voudourisite is biaxial (–) with refractive indices at 589(1) nm of α = 1.580(2), β = 1.624(2), γ = 1.640(2), 2Vmeas. = 70(5)°, 2Vcalc. = 61° and β ~ || [010]. Lazaridisite is biaxial neutral with refractive indices at 589(1) nm of α = 1.552(2), β = 1.561(2), γ = 1.570(2), 2Vmeas. = 90(5)° and 2Vcalc. = 90°. The chemical compositions of voudourisite and lazaridisite are close to ideal with only minor amounts of copper detectable. The atomic arrangement in voudourisite (space group P21/c with a = 7.633(2), b = 7.458(2), c = 8.151(2) Å, β = 122.35(1)° and V = 392.0(2) Å3) is related to that of kieserite while lazaridisite (space group C2/c with a = 14.813(3), b = 11.902(2), c = 9.466(2) Å, β = 97.38(1)° and V = 1655.2(6) Å3) is a distinct structure-type. Calculated densities are 3.838 and 3.088 g/cm3, respectively.
The strongest lines in the powder X-ray pattern [d in Å (I) (hkl)] are: 4.890 (66) (110); 3.741 (25) (020); 3.578 (100) (11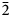 $\bar 2$); 3.230 (43) (200); 2.525 (33) (022); 2.395 (29) (112) for voudourisite and 6.860 (100) (200); 6.317 (72) (111); 5.965 (84) (020); 4.512 (58) (310); 3.727 (78) (202); 3.608(82)(13
$\bar 2$); 3.230 (43) (200); 2.525 (33) (022); 2.395 (29) (112) for voudourisite and 6.860 (100) (200); 6.317 (72) (111); 5.965 (84) (020); 4.512 (58) (310); 3.727 (78) (202); 3.608(82)(13 $\bar 1$); 3.109 (83) (40
$\bar 1$); 3.109 (83) (40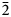 $\bar 2$); 3.020 (50) (33
$\bar 2$); 3.020 (50) (33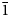 $\bar 1$) for lazaridisite, respectively.
$\bar 1$) for lazaridisite, respectively.
Associate Editor: Michael Rumsey