Article contents
Uroxite and metauroxite, the first two uranyl oxalate minerals
Published online by Cambridge University Press: 02 September 2019
Abstract
Uroxite (IMA2018-100), [(UO2)2(C2O4)(OH)2(H2O)2]⋅H2O, and metauroxite (IMA2019-030), (UO2)2(C2O4)(OH)2(H2O)2, are the first two uranyl oxalate minerals. Uroxite was found in the Markey mine, Red Canyon, San Juan County, Utah, USA and in the Burro mine, Slick Rock district, San Miguel County, Colorado, USA. Metauroxite was found only in the Burro mine. Both minerals are post-mining secondary phases found in efflorescent crusts on mine walls. Uroxite occurs as light yellow striated blades exhibiting moderate neon-green fluorescence, ca 2 Mohs hardness with good {101} and {010} cleavages. Calculated density = 4.187 g/cm3. Optics are: biaxial (–), α = 1.602(2), β = 1.660(2), γ = 1.680(2) (white light), 2Vmeas. = 59(1)°, 2Vcalc = 59.1°, moderate r > v dispersion, orientation Y = b, Z ∧ a = 35° in obtuse β and it is nonpleochroic. Metauroxite occurs as light yellow crude blades and tablets exhibiting weak green–grey fluorescence, ca 2 Mohs hardness with good {001} cleavage. Calculated density = 4.403 g/cm3. Approximate optics are: α′ = 1.615(5) and γ′ = 1.685(5). Electron probe microanalysis provided UO3 79.60, C2O3 10.02, H2O 10.03, total 99.65 wt.% for uroxite and UO3 82.66, C2O3 10.40, H2O 7.81, total 100.87 wt.% for metauroxite; C2O3 and H2O are based on the structures. Uroxite is monoclinic, P21/c, a = 5.5698(2), b = 15.2877(6), c = 13.3724(9) Å, β = 94.015(7)°, V = 1135.86(10) Å3 and Z = 4. Metauroxite is triclinic, P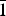 ${\bar 1}$, a = 5.5635(3), b = 6.1152(4), c = 7.8283(4) Å, α = 85.572(5), β = 89.340(4), γ = 82.468°, V = 263.25(3) Å3 and Z = 1. The strongest reflections of the powder XRD pattern [d, Å (I, %)(hkl)] are for uroxite: 10.05(38)(011), 5.00(100)(022,
${\bar 1}$, a = 5.5635(3), b = 6.1152(4), c = 7.8283(4) Å, α = 85.572(5), β = 89.340(4), γ = 82.468°, V = 263.25(3) Å3 and Z = 1. The strongest reflections of the powder XRD pattern [d, Å (I, %)(hkl)] are for uroxite: 10.05(38)(011), 5.00(100)(022, 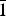 ${\bar 1}$11), 4.75(23)(031), 4.43(51)(120,
${\bar 1}$11), 4.75(23)(031), 4.43(51)(120, 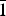 ${\bar 1}$02), 3.567(33)(131), 3.341(29)(033,
${\bar 1}$02), 3.567(33)(131), 3.341(29)(033, 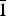 ${\bar 1}$32, 004), 2.623(28)(
${\bar 1}$32, 004), 2.623(28)( ${\bar 2}$02, 015,
${\bar 2}$02, 015, 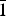 ${\bar 1}$43, 220) and for metauroxite: 6.06(45)(010), 5.52(33)(100), 4.97(34)(011), 4.52(100)(0
${\bar 1}$43, 220) and for metauroxite: 6.06(45)(010), 5.52(33)(100), 4.97(34)(011), 4.52(100)(0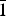 ${\bar 1}$1, 101), 3.888(80)(111, 002,
${\bar 1}$1, 101), 3.888(80)(111, 002, 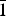 ${\bar 1}$10), 3.180(51)(
${\bar 1}$10), 3.180(51)(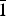 ${\bar 1}$02, 0
${\bar 1}$02, 0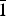 ${\bar 1}$2), 2.604(32)(
${\bar 1}$2), 2.604(32)(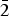 ${\bar 2}$01,
${\bar 2}$01, 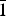 ${\bar 1}$
${\bar 1}$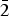 ${\bar 2}$1). In the structure of uroxite (R1 = 0.0333 for 2081 I > 2σI reflections), UO7 pentagonal bipyramids share corners forming [U4O24] tetramers, which are linked by C2O4 groups to form corrugated sheets. In the structure of metauroxite (R1 = 0.0648 for 1602 I > 2σI reflections) UO7 pentagonal bipyramids share edges forming [U2O12] dimers, which are linked by C2O4 groups to form zigzag chains.
${\bar 2}$1). In the structure of uroxite (R1 = 0.0333 for 2081 I > 2σI reflections), UO7 pentagonal bipyramids share corners forming [U4O24] tetramers, which are linked by C2O4 groups to form corrugated sheets. In the structure of metauroxite (R1 = 0.0648 for 1602 I > 2σI reflections) UO7 pentagonal bipyramids share edges forming [U2O12] dimers, which are linked by C2O4 groups to form zigzag chains.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2019
Footnotes
Associate Editor: Peter Leverett
References
- 12
- Cited by


