Published online by Cambridge University Press: 16 March 2021
Thebaite-(NH4), (NH4,K)3Al(C2O4)(PO3OH)2(H2O), is a new mineral species (IMA2020-072) from the Rowley mine, Maricopa County, Arizona, USA. It occurs in an unusual bat-guano-related, post-mining assemblage of phases that include a variety of vanadates, phosphates, oxalates and chlorides, some containing NH4+. Other secondary minerals found in association with thebaite-(NH4) are antipinite, vanadinite and at least one other new mineral. Crystals of thebaite-(NH4) are colourless blades up to ~0.1 mm in length. The streak is white, lustre is vitreous, Mohs hardness is 1½–2, tenacity is brittle and fracture is splintery. There are two good cleavages in the [010] zone, probably {100} and {10$\bar{2}$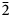 }. The calculated density is 2.093 g⋅cm–3. Thebaite-(NH4) is optically biaxial (–) with α = 1.490(2), β = 1.534(2), γ = 1.570(2) (white light); 2V = 82.7(5)°; slight r > v dispersion; and orientation X = b, Y ^ c = 13° in obtuse β. Electron microprobe analysis gave the empirical formula [(NH4)2.12K0.69Na0.20]Σ3.01(Al0.84Fe3+0.11V3+0.04)Σ0.99(C2O4)[(P0.98Si0.02)O3OH]2(H2O), with the C, N and H contents constrained by the crystal structure. Raman spectroscopy confirmed the presence of NH4 and C2O4. Thebaite-(NH4) is monoclinic, P21/c, with a = 11.156(9), b = 6.234(6), c = 18.651(16) Å, β = 102.928(15)°, V = 1264.2(19) Å3 and Z = 4. The structural unit in the crystal structure of thebaite-(NH4) (R1 = 0.0612 for 863 Io > 2σI reflections) is a double-strand chain of corner-sharing AlO6 octahedra and PO3OH tetrahedra decorated by additional PO3OH tetrahedra and C2O4 groups. The decorated chains connect to one another through bonds to NH4+ and K+ and through hydrogen bonds.
}. The calculated density is 2.093 g⋅cm–3. Thebaite-(NH4) is optically biaxial (–) with α = 1.490(2), β = 1.534(2), γ = 1.570(2) (white light); 2V = 82.7(5)°; slight r > v dispersion; and orientation X = b, Y ^ c = 13° in obtuse β. Electron microprobe analysis gave the empirical formula [(NH4)2.12K0.69Na0.20]Σ3.01(Al0.84Fe3+0.11V3+0.04)Σ0.99(C2O4)[(P0.98Si0.02)O3OH]2(H2O), with the C, N and H contents constrained by the crystal structure. Raman spectroscopy confirmed the presence of NH4 and C2O4. Thebaite-(NH4) is monoclinic, P21/c, with a = 11.156(9), b = 6.234(6), c = 18.651(16) Å, β = 102.928(15)°, V = 1264.2(19) Å3 and Z = 4. The structural unit in the crystal structure of thebaite-(NH4) (R1 = 0.0612 for 863 Io > 2σI reflections) is a double-strand chain of corner-sharing AlO6 octahedra and PO3OH tetrahedra decorated by additional PO3OH tetrahedra and C2O4 groups. The decorated chains connect to one another through bonds to NH4+ and K+ and through hydrogen bonds.
Associate Editor: Peter Leverett