Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
KUSACHI, Isao
HENMI, Chiyoko
and
KOBAYASHI, Shoichi
1995.
Frolovite from Fuka, Okayama Prefecture, Japan..
Mineralogical Journal,
Vol. 17,
Issue. 7,
p.
330.
KUSACHI, Isao
HENMI, Chiyoko
and
KOBAYASHI, Shoichi
1996.
Johnbaumite from Fuka, Okayama Prefecture, Japan..
Mineralogical Journal,
Vol. 18,
Issue. 2,
p.
60.
KUSACHI, Isao
HENMI, Chiyoko
and
KOBAYASHI, Shoichi
1997.
Sibirskite from Fuka, Okayama Prefecture, Japan..
Mineralogical Journal,
Vol. 19,
Issue. 3,
p.
109.
KUSACHI, Isao
TAKECHI, Yasushi
HENMI, Chiyoko
and
KOBAYASHI, Shoichi
1997.
Borcarite from Fuka, Okayama Prefecture, Japan..
Mineralogical Journal,
Vol. 19,
Issue. 3,
p.
115.
Matsubara, Satoshi
Miyawaki, Ritsuro
Kato, Akira
Yokoyama, Kazumi
and
Okamoto, Akiyoshi
1998.
Okayamalite, Ca2B2SiO7, a new mineral, boron analogue of gehlenite.
Mineralogical Magazine,
Vol. 62,
Issue. 5,
p.
703.
Kusachi, I.
Takechi, Y.
Henmi, C.
and
Kobayashi, S.
1998.
Parasibirskite, a new mineral from Fuka, Okayama Prefecture, Japan.
Mineralogical Magazine,
Vol. 62,
Issue. 4,
p.
521.
KUSACHI, Isao
TAKECHI, Yasushi
KOBAYASHI, Shoichi
YAMAKAWA, Junji
NAKAMUTA, Yoshihiro
LEE, Kyue-Hyung
and
MOTOMIZU, Shoji
1999.
Hexahydroborite from Fuka, Okayama Prefecture, Japan..
Mineralogical Journal,
Vol. 21,
Issue. 1,
p.
9.
KUSACHI, Isao
SHIRAGA, Kanako
KOBAYASHI, Shoichi
YAMAKAWA, Junji
and
TAKECHI, Yasushi
2000.
Uralborite from Fuka, Okayama Prefecture, Japan..
Journal of Mineralogical and Petrological Sciences,
Vol. 95,
Issue. 4,
p.
43.
KUSACHI, Isao
NISHIMURA, Makoto
SHIRAGA, Kanako
KOBAYASHI, Shoichi
and
YAMAKAWA, Junji
2001.
Kinoite from Fuka, Okayama Prefecture, Japan..
Journal of Mineralogical and Petrological Sciences,
Vol. 96,
Issue. 1,
p.
29.
Wu, L
Chen, X.L
Tu, Q.Y
He, M
Zhang, Y
and
Xu, Y.P
2003.
Phase relations in the system Li2O–CaO–B2O3.
Journal of Alloys and Compounds,
Vol. 358,
Issue. 1-2,
p.
23.
Zhang, Shuyu
Wu, Xing
Song, Youting
Ni, Daiqin
Hu, Boqing
and
Zhou, Tang
2003.
Growth of birefringent Ca3(BO3)2 crystals by the Czochralski method.
Journal of Crystal Growth,
Vol. 252,
Issue. 1-3,
p.
246.
SATISH-KUMAR, Madhusoodhan
YOSHIDA, Yasuhito
and
KUSACHI, Isao
2004.
The role of aqueous silica concentration in controlling the mineralogy during high temperature contact metamorphism: A case study from Fuka contact aureole, Okayama, Japan.
Journal of Mineralogical and Petrological Sciences,
Vol. 99,
Issue. 5,
p.
328.
KUSACHI, Isao
KOBAYASHI, Shoichi
TANABE, Mitsuo
KISHI, Shigetomo
and
YAMAKAWA, Junji
2004.
Inyoite from Fuka, Okayama Prefecture, Japan.
Journal of Mineralogical and Petrological Sciences,
Vol. 99,
Issue. 2,
p.
67.
Lu, Xiuai
You, Zhenyu
Li, Jianfu
Zhu, Zhaojie
Jia, Guohua
Wang, Yan
Wu, Baichang
and
Tu, Chaoyang
2005.
Growth and properties of pure and rare earth-doped Ca3 (BO3)2 single crystal.
Journal of Crystal Growth,
Vol. 281,
Issue. 2-4,
p.
416.
Kato, Akihiko
and
Rikukawa, Hiroshi
2005.
First-principles studies of large birefringences in alkaline-earth orthoborate crystals.
Physical Review B,
Vol. 72,
Issue. 4,
TAKADA, Masayuki
KUSACHI, Isao
KISHI, Shigetomo
TANABE, Mitsuo
and
YASUDA, Takashi
2005.
Crystal forms of borate minerals from the Fuka mine, Okayama Prefecture, Japan.
Japanese Magazine of Mineralogical and Petrological Sciences,
Vol. 34,
Issue. 5,
p.
252.
Aleksandrov, S. M.
2007.
Endogenous transformations of kotoite in calciphyres at magnesian-skarn deposits of boron.
Geochemistry International,
Vol. 45,
Issue. 7,
p.
666.
KUSACHI, Isao
SHIRAISHI, Naoko
SHIMADA, Kazumasa
OHNISHI, Masayuki
and
KOBAYASHI, Shoichi
2008.
CO3-rich charlesite from the Fuka mine, Okayama Prefecture, Japan.
Journal of Mineralogical and Petrological Sciences,
Vol. 103,
Issue. 1,
p.
47.
OHNISHI, Masayuki
2009.
Numanoite discovered as a new mineral species from the Fuka mine, Okayama Prefecture, Japan.
Japanese Magazine of Mineralogical and Petrological Sciences,
Vol. 38,
Issue. 1,
p.
25.
TAKAHASHI, Ryosuke
KUSACHI, Isao
and
MIURA, Hiroyuki
2010.
Crystal structure of parasibirskite (CaHBO3) and polymorphism in sibirskite and parasibirskite.
Journal of Mineralogical and Petrological Sciences,
Vol. 105,
Issue. 2,
p.
70.
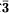 c, a = 8.638(1), c = 11.850(2)Å, Z = 6. The strongest lines in the X-ray powder pattern [d in A(I)(hkl)] are 2.915(100)(113), 1.895(75)(223), 2.756(61)(104), 2.493(44)(300), 2.044(21) (214,131), 2.160(19)(220), 1.976(18)(006), 1.549(12)(306). The Vickers microhardness is 478 kg mm−2 and the Mohs hardness 4.5. The density is 3.10(2) g cm−3 (meas.) and 3.11 g cm−3 (calc.). Wet chemical analyses give the values CaO 71.13%, B2O3 28.41%, ig. loss 0.14%, and total 99.68%. The empirical formula calculated on the basis of O = 6 is Ca3.053B1.965O6, with the simplified formula of Ca3B2O6.
c, a = 8.638(1), c = 11.850(2)Å, Z = 6. The strongest lines in the X-ray powder pattern [d in A(I)(hkl)] are 2.915(100)(113), 1.895(75)(223), 2.756(61)(104), 2.493(44)(300), 2.044(21) (214,131), 2.160(19)(220), 1.976(18)(006), 1.549(12)(306). The Vickers microhardness is 478 kg mm−2 and the Mohs hardness 4.5. The density is 3.10(2) g cm−3 (meas.) and 3.11 g cm−3 (calc.). Wet chemical analyses give the values CaO 71.13%, B2O3 28.41%, ig. loss 0.14%, and total 99.68%. The empirical formula calculated on the basis of O = 6 is Ca3.053B1.965O6, with the simplified formula of Ca3B2O6.