Published online by Cambridge University Press: 05 July 2018
Schlüterite-(Y), ideally (Y,REE)2Al(Si2O7)(OH)2F, is a new silicate mineral species from the Stetind pegmatite, Tysfjord, Nordland, Norway. It forms dense, fibrous, radiating aggregates (up to ∼2 mm) diverging to individual needle-like crystals (up to ∼1 mm long) in cavities. Crystals are acicular to bladed, flattened on {001} and elongated along [010], and the dominant form is {001}. Schlüterite-(Y) is transparent, pale pink with a white streak and a vitreous lustre, and does not fluoresce under short-wave ultraviolet light. Mohs hardness is 5½–6, and schlüterite-(Y) is brittle with an irregular fracture, and has no cleavage. The calculated density is 4.644 g/cm3. The indices of refraction are α = 1.755, β = 1.760, γ = 1.770, all ± 0.005, 2Vobs = 71.8 (5)°, 2Vcalc = 71°, non-pleochroic, optic orientation is X ˆ a = 83.1° (β obtuse), Y//b, Z ˆ c = 50.3° (β acute). Schlüterite-(Y) is monoclinic, space group P21/c, a 7.0722(2), b 5.6198(1), c 21.4390(4) Å, β 122.7756(3)°, V 716.43(5) Å3, Z = 4. The seven strongest lines in the X-ray powder-diffraction pattern are as follows: [d (Å), I, (hkl)]: 4.769, 100, (012); 2.972, 55, (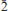 14); 3.289, 51, (112); 2.728, 49, (
14); 3.289, 51, (112); 2.728, 49, (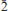 16); 2.810, 37, (020); 3.013, 37, ((
16); 2.810, 37, (020); 3.013, 37, ((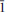 16); 4.507, 36, (004). Chemical analysis by electron microprobe gave SiO2 22.64, Al2O3 9.45, Y2O3 15.35, La2O3 3.25, Ce2O3 9.69, Pr2O3 2.05, Nd2O3 9.50, Sm2O3 3.57, Gd2O3 4.65, Dy2O3 4.21, Er2O3 2.31, Yb2O3 1.86, F 2.71, H2Ocalc 3.78, O = F −1.14, sum 93.88 wt%. The H2O content was determined by crystal-structure analysis. On the basis of 10 anions with (OH) + F = 3 a.p.f.u. (atoms per formula unit), the empirical formula is (Y0.73Ce0.32Nd0.30Gd0.14Dy0.12La0.11Sm0.11Pr0.07Er0.06Yb0.05)Σ=2.01Al0.99Si2.01O7(OH)2.24F0.76. The crystal structure of schlüterite-(Y) was solved by direct methods and refined to an R1 index of 1.8% based on 1422 unique observed reflections. In the structure of schlüterite-(Y), Al(OH)4O2 octahedra share (OH)–(OH) edges to form [MΦ4] chains that are decorated by (Si2O7) groups that bridge O vertices of neighbouring octahedra in a staggered fashion on either side of the chain. These [Al(OH)2(Si2O7)] chains extend parallel to b, and are linked into a continuous framework via bonds to interstitial [8](Y,REE) (= <2.400 Å>) and [9](Y,REE) (= <2.548 Å>) atoms.
16); 4.507, 36, (004). Chemical analysis by electron microprobe gave SiO2 22.64, Al2O3 9.45, Y2O3 15.35, La2O3 3.25, Ce2O3 9.69, Pr2O3 2.05, Nd2O3 9.50, Sm2O3 3.57, Gd2O3 4.65, Dy2O3 4.21, Er2O3 2.31, Yb2O3 1.86, F 2.71, H2Ocalc 3.78, O = F −1.14, sum 93.88 wt%. The H2O content was determined by crystal-structure analysis. On the basis of 10 anions with (OH) + F = 3 a.p.f.u. (atoms per formula unit), the empirical formula is (Y0.73Ce0.32Nd0.30Gd0.14Dy0.12La0.11Sm0.11Pr0.07Er0.06Yb0.05)Σ=2.01Al0.99Si2.01O7(OH)2.24F0.76. The crystal structure of schlüterite-(Y) was solved by direct methods and refined to an R1 index of 1.8% based on 1422 unique observed reflections. In the structure of schlüterite-(Y), Al(OH)4O2 octahedra share (OH)–(OH) edges to form [MΦ4] chains that are decorated by (Si2O7) groups that bridge O vertices of neighbouring octahedra in a staggered fashion on either side of the chain. These [Al(OH)2(Si2O7)] chains extend parallel to b, and are linked into a continuous framework via bonds to interstitial [8](Y,REE) (= <2.400 Å>) and [9](Y,REE) (= <2.548 Å>) atoms.