Article contents
Redefinition of thérèsemagnanite, NaCo4(SO4)(OH)6Cl·6H2O: new data and relationship to ‘cobaltogordaite’
Published online by Cambridge University Press: 28 February 2018
Abstract
Thérèsemagnanite was originally described from the Cap Garonne mine, Var, France. Its ideal formula was reported as (Co,Zn,Ni)6(SO4)(OH,Cl)10·8H2O; without crystal structure data, only the powder X-ray diffraction pattern was given. Revision of the holotype material revealed that thérèsemagnanite is identical to ‘cobaltogordaite’ (IMA2014-043), recently described from the Blue Lizard mine, Utah, USA. Thérèsemagnanite is thus redefined in accordance with the new data obtained for the neotype specimen from Blue Lizard (formerly the holotype specimen of ‘cobaltogordaite’) and ‘cobaltogordaite’ has been discredited by the International Mineralogical Association Commission on New Mineral Nomenclature and Classification (IMA CNMNC). Thérèsemagnanite has the ideal, end-member formula NaCo4(SO4)(OH)6Cl·6H2O. The empirical formulae of the holotype (Cap Garonne) and the neotype (Blue Lizard), both based on microprobe analyses and calculated on the basis of 17 O + Cl atoms per formula unit (with fixed 6 OH groups and 6 H2O molecules; H content is calculated by stoichiometry) are (Na0.64K0.09)Σ0.73(Co2.35Zn1.22Ni0.50)Σ4.07S1.02O3.98(OH)6Cl1.02·6H2O and Na1.01(Co1.90Zn1.37Ni0.48Cu0.15Mn0.05)Σ3.95S1.03O4.09(OH)6Cl0.91·6H2O, respectively. Thérèsemagnanite is trigonal, P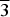 $\overline 3 $, a = 8.349(3), c = 13.031(2) Å, V = 786.6(4) Å3 and Z = 2 (neotype). The strongest powder X-ray diffraction lines are [dobs in Å (hkl) (Irel)]: 13.10 (001)(100), 6.53 (002)(8), 4.173 (110)(4), 3.517 (112)(5), 2.975 (104, 10
$\overline 3 $, a = 8.349(3), c = 13.031(2) Å, V = 786.6(4) Å3 and Z = 2 (neotype). The strongest powder X-ray diffraction lines are [dobs in Å (hkl) (Irel)]: 13.10 (001)(100), 6.53 (002)(8), 4.173 (110)(4), 3.517 (112)(5), 2.975 (104, 10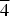 $\overline 4 $)(4), 2.676 (211)(5) and 2.520 (12
$\overline 4 $)(4), 2.676 (211)(5) and 2.520 (12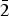 $\bar 2$)(5) (neotype). Thérèsemagnanite is a cobalt analogue of gordaite, NaZn4(SO4)(OH)6Cl·6H2O. These minerals represent the gordaite group, accepted by the IMA CNMNC.
$\bar 2$)(5) (neotype). Thérèsemagnanite is a cobalt analogue of gordaite, NaZn4(SO4)(OH)6Cl·6H2O. These minerals represent the gordaite group, accepted by the IMA CNMNC.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: Stuart Mills
References
- 5
- Cited by


