Article contents
Reaphookhillite, MgZn2(PO4)2⋅4H2O, the Mg analogue of parahopeite from Reaphook Hill, South Australia
Published online by Cambridge University Press: 18 February 2022
Abstract
Reaphookhillite, ideally MgZn2(PO4)2⋅4H2O, is a new phosphate mineral from Reaphook Hill, Flinders Ranges, South Australia, Australia. Reaphookhillite occurs as colourless, bladed to thin tabular crystals to 0.6 mm across. Cleavage is perfect parallel to {010}. The mineral occur as overgrowths on parahopeite crystals and is associated with scholzite, leucophosphite and chalcophanite. The calculated density is 3.09 g/cm3 from the empirical formula. Reaphookhillite is optically biaxial (+), α = 1.583(3), β = 1.596(3), γ = 1.611(3) and 2Vcalc = 88.7°. Electron microprobe analyses gave ZnO 41.57, MgO 7.96, MnO 0.40, P2O5 33.72, H2O(calc) 16.92, total 100.57 wt.%. The empirical formula, based on 12 O apfu, is Mg0.83Zn2.16Mn2+0.02(PO4)2.01⋅3.97H2O. Reaphookhillite is triclinic, P${\bar 1}$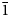 , with the unit-cell parameters of a = 5.7588(12), b = 7.5341(15) c = 5.2786(11) Å, α = 93.44(3), β = 91.27(3), γ = 91.30(3)°, V = 228.49(8) Å3 and Z = 1. The strongest eight lines in the powder X-ray diffraction pattern are [dobs in Å (I) (hkl)] 7.577 (100) (010); 4.461 (24) (01${\bar 1}$
, with the unit-cell parameters of a = 5.7588(12), b = 7.5341(15) c = 5.2786(11) Å, α = 93.44(3), β = 91.27(3), γ = 91.30(3)°, V = 228.49(8) Å3 and Z = 1. The strongest eight lines in the powder X-ray diffraction pattern are [dobs in Å (I) (hkl)] 7.577 (100) (010); 4.461 (24) (01${\bar 1}$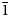 ); 4.461 (24) (01${\bar 1}$
); 4.461 (24) (01${\bar 1}$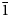 ); 3.771 (14) (020); 3.158 (13) (02${\bar 1}$
); 3.771 (14) (020); 3.158 (13) (02${\bar 1}$ ); 2.982 (32) (021); 2.880 (27) (200); 2.775 (14) (1${\bar 2}$
); 2.982 (32) (021); 2.880 (27) (200); 2.775 (14) (1${\bar 2}$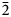 1, 12${\bar 1}$
1, 12${\bar 1}$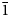 ); and 2.668 (13) (1${\bar 2}{\bar 1}$
); and 2.668 (13) (1${\bar 2}{\bar 1}$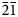 , 210). Reaphookhillite is isostructural with parahopeite, with Mg replacing Zn in the 6-coordinated site in the structure. The structure contains ZnO4 and PO4 tetrahedra which share corners to form a sheet in the (001) plane. Sheets are linked in the c direction by corner sharing MgO2(H2O)4 octahedra.
, 210). Reaphookhillite is isostructural with parahopeite, with Mg replacing Zn in the 6-coordinated site in the structure. The structure contains ZnO4 and PO4 tetrahedra which share corners to form a sheet in the (001) plane. Sheets are linked in the c direction by corner sharing MgO2(H2O)4 octahedra.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Anthony R Kampf
This paper is part of a thematic set that honours the contributions of Peter Williams.
References
- 2
- Cited by


