Article contents
The new mineral zolotarevite, Na5Zr[Si6O15(ОН)3]⋅2–3H2O, the first highly hydrated lovozerite-group member from the Lovozero alkaline massif, Kola Peninsula, Russia
Published online by Cambridge University Press: 07 March 2022
Abstract
Zolotarevite (IMA2020-076), ideally Na5Zr[Si6O15(ОН)3]⋅2–3H2O, is а new mineral of the lovozerite group. The mineral was found in a leucocratic nepheline syenite from Kedykverpakhk Mt., Lovozero alkaline massif, Kola Peninsula, Russia. It occurs as anhedral grains up to 1 mm across and is associated with microcline–perthite, nepheline, sodalite, aegirine, lamprophyllite, lueshite, umbozerite, lomonosovite, nastrophite, a mineral of the kazakovite–tisinalite series, sphalerite and löllingite. Zolotarevite is cherry red, with a vitreous lustre and white streak. The mineral is brittle, with a Mohs hardness of 5. Cleavage was not observed; the fracture is uneven. The measured density is 2.75(5) g⋅cm–3, the density calculated using the empirical formula and single-crystal unit-cell parameters is 2.85 g⋅cm–3. Zolotarevite is anomalously biaxial (–), α = 1.580(2), β = 1.600(2) and γ = 1.602(2) (for λ = 589 nm); 2Vmeas < 10°. Chemical data (electron microprobe, wt.%) are: Na2O 20.41, СаО 0.42, MnO 3.49, Fе2O3 0.55, SiO2 52.46, ТiO2 1.34, ZrO2 11.33, H2O (calculated from the structural formula) 10.20, total 100.20. The empirical formula based оn 6 Si atoms per formula unit is Na4.53Zr0.63Mn0.34Ti0.11Ca0.05Fe3+0.05Si6O14.43(ОН)3.56(H2О)2.11. Zolotarevite is trigonal, space group R${\bar 3}$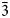 m, with a = 10.294(6) Å, c = 13.115(8) Å, V = 1203.7(16) Å3 and Z = 3. The crystal structure was solved from single-crystal data and refined to R1 = 0.049. The strongest lines of the powder X-ray diffraction pattern [d in Å (I) (hkl)] are: 7.37 (69) (101); 5.26 (56) (012); 3.686 (64) (202); 3.330 (79) (113); 3.265 (99) (211), 2.640 (100) (024) and 2.576 (60) (220). Zolotarevite is unique in that it is a highly hydrated lovozerite-group mineral with the B site occupied by variable amounts of H2O.
m, with a = 10.294(6) Å, c = 13.115(8) Å, V = 1203.7(16) Å3 and Z = 3. The crystal structure was solved from single-crystal data and refined to R1 = 0.049. The strongest lines of the powder X-ray diffraction pattern [d in Å (I) (hkl)] are: 7.37 (69) (101); 5.26 (56) (012); 3.686 (64) (202); 3.330 (79) (113); 3.265 (99) (211), 2.640 (100) (024) and 2.576 (60) (220). Zolotarevite is unique in that it is a highly hydrated lovozerite-group mineral with the B site occupied by variable amounts of H2O.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Ian T. Graham
References
- 4
- Cited by


