Published online by Cambridge University Press: 28 February 2018
Melcherite (IMA2015-018), ideally Ba2Na2Mg[Nb6O19]·6H2O, occurs as a vug mineral in the carbonatite of the Jacupiranga mine, Cajati county, São Paulo state, Brazil, associated with dolomite, calcite, magnetite, pyrrhotite, tochilinite, ‘pyrochlore’ and fluorapatite. This is also the type locality for zirkelite, quintinite, menezesite and pauloabibite. The mineral forms irregular, tabular crystals up to 200 µm in maximum dimension. Melcherite is transparent and displays a vitreous lustre; it is beige with a white streak. It is non-fluorescent. The mineral displays perfect cleavage on {001}. Chemical composition varies from Ba2Na2Mg[Nb6O19].6H2O to (BaK)(NaCa)Mg[Nb6O19].6H2O. Empirical formulae for the first and the second compositions are: (Ba1.75K0.19)Σ1.94(Na1.80Ca0.19)Σ1.99(Mg0.96Mn0.02Al0.02)Σ1.00Nb6.02O19.00·6H2O and (Ba0.99K1.00)Σ1.99(Na1.02Ca0.96)Σ1.98(Mg0.95Mn0.05)Σ1.00Nb6.02O19.00·6H2O, respectively. Data for a single crystal with the second composition are: trigonal, R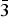 $\bar 3$, a = 9.0117(6) Å, c = 23.3986(16) Å, V = 1645.64(19) Å3 and Z = 3. Calculated density for this formula is 3.733 g/cm3, and the calculated mean refractive index is 1.924. Melcherite is a hexaniobate that has structural layers parallel to the xy plane that stack along the c axis with simultaneous 1/3 [110] displacement so as to produce an R lattice. The melcherite structure is built by layers of [(Ba,K)(O,H2O)9] polyhedra and the [Nb6O19]8− super-octahedron (Lindqvist anion) interconnected by [(Na,Ca)O6] polyhedra. Cations of Mg2+ are bonded to six water molecules each and are not associated with Lindqvist oxygen ions. The mineral is named in honour of Geraldo Conrado Melcher (1924–2011), a pioneer in Jacupiranga carbonatite studies.
$\bar 3$, a = 9.0117(6) Å, c = 23.3986(16) Å, V = 1645.64(19) Å3 and Z = 3. Calculated density for this formula is 3.733 g/cm3, and the calculated mean refractive index is 1.924. Melcherite is a hexaniobate that has structural layers parallel to the xy plane that stack along the c axis with simultaneous 1/3 [110] displacement so as to produce an R lattice. The melcherite structure is built by layers of [(Ba,K)(O,H2O)9] polyhedra and the [Nb6O19]8− super-octahedron (Lindqvist anion) interconnected by [(Na,Ca)O6] polyhedra. Cations of Mg2+ are bonded to six water molecules each and are not associated with Lindqvist oxygen ions. The mineral is named in honour of Geraldo Conrado Melcher (1924–2011), a pioneer in Jacupiranga carbonatite studies.
Associate Editor: Ian Graham
Deceased July 2014