Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kampf, A. R.
Plášil, J.
Kasatkin, A. V.
and
Marty, J.
2014.
Belakovskiite,
Na7(UO2)(SO4)4(SO3OH)(H2O)3,
a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County,
Utah, USA.
Mineralogical Magazine,
Vol. 78,
Issue. 3,
p.
639.
Kampf, Anthony R.
Plášil, Jakub
Kasatkin, Anatoly V.
Marty, Joe
and
Čejka, Jiří
2015.
Fermiite, Na4(UO2)(SO4)3·3H2O and oppenheimerite, Na2(UO2)(SO4)2·3H2O, two new uranyl sulfate minerals from the Blue Lizard mine, San Juan County, Utah, USA.
Mineralogical Magazine,
Vol. 79,
Issue. 5,
p.
1123.
Kampf, Anthony R.
Plášil, Jakub
Kasatkin, Anatoly V.
and
Marty, Joe
2015.
Bobcookite, NaAl(UO2)2(SO4)4·18H2O and wetherillite, Na2Mg(UO2)2(SO4)4·18H2O, two new uranyl sulfate minerals from the Blue Lizard mine, San Juan County, Utah, USA.
Mineralogical Magazine,
Vol. 79,
Issue. 3,
p.
695.
Belogub, Elena V.
Krivovichev, Sergey V.
Pekov, Igor V.
Kuznetsov, Aleksey M.
Yapaskurt, Vasiliy O.
Kotlyarov, Vasiliy A.
Chukanov, Nikita V.
and
Belakovskiy, Dmitry I.
2015.
Nickelpicromerite, K2Ni(SO4)2•6H2O, a new picromerite-group mineral from Slyudorudnik, South Urals, Russia.
Mineralogy and Petrology,
Vol. 109,
Issue. 2,
p.
143.
Plášil, J.
Hloušek, J.
Kasatkin, A. V.
Škoda, R.
Novák, M.
and
Čejka, J.
2015.
Geschieberite, K2(UO2)(SO4)2(H2O)2, a new uranyl sulfate mineral from Jáchymov.
Mineralogical Magazine,
Vol. 79,
Issue. 1,
p.
205.
Sharifironizi, Melika
Szymanowski, Jennifer E.S.
Sigmon, Ginger E.
Navrotsky, Alexandra
Fein, Jeremy B.
and
Burns, Peter C.
2016.
Thermodynamic studies of zippeite, a uranyl sulfate common in mine wastes.
Chemical Geology,
Vol. 447,
Issue. ,
p.
54.
Felder, Justin
Yeon, Jeongho
Smith, Mark
and
zur Loye, Hans-Conrad
2017.
Application of a mild hydrothermal method to the synthesis of mixed transition-metal(ii)/uranium(iv) fluorides.
Inorganic Chemistry Frontiers,
Vol. 4,
Issue. 2,
p.
368.
Felder, Justin B.
Smith, Mark D.
and
zur Loye, Hans-Conrad
2017.
Supercritical synthesis and topological analysis of K5U5O17(OH).
CrystEngComm,
Vol. 19,
Issue. 25,
p.
3499.
Kampf, Anthony R.
Plášil, Jakub
Kasatkin, Anatoly V.
Marty, Joe
and
Čejka, Jiří
2017.
Klaprothite, péligotite and ottohahnite, three new minerals with bidentate UO7–SO4linkages from the Blue Lizard mine, San Juan County, Utah, USA.
Mineralogical Magazine,
Vol. 81,
Issue. 4,
p.
753.
Kampf, Anthony R.
Plášil, Jakub
Čejka, Jiří
Marty, Joe
Škoda, Radek
and
Lapčák, Ladislav
2017.
Alwilkinsite-(Y), a new rare-earth uranyl sulfate mineral from
the Blue Lizard mine, San Juan County, Utah, USA.
Mineralogical Magazine,
Vol. 81,
Issue. 4,
p.
895.
Krivovichev, Sergey V.
Meisser, Nicolas
Brugger, Joel
Chernyshov, Dmitry V.
and
Gurzhiy, Vladislav V.
2018.
Synchrotron Diffraction Study of the Crystal Structure of Ca(UO2)6(SO4)2O2(OH)6·12H2O, a Natural Phase Related to Uranopilite.
Minerals,
Vol. 8,
Issue. 12,
p.
569.
Lu, Grace
Haes, Amanda J.
and
Forbes, Tori Z.
2018.
Detection and identification of solids, surfaces, and solutions of uranium using vibrational spectroscopy.
Coordination Chemistry Reviews,
Vol. 374,
Issue. ,
p.
314.
Gurzhiy, Vladislav V.
and
Plášil, Jakub
2019.
Structural complexity of natural uranyl sulfates.
Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials,
Vol. 75,
Issue. 1,
p.
39.
Nazarchuk, Evgeny
Charkin, Dmitri
Siidra, Oleg
and
Kalmykov, Stepan
2020.
Organically Templated Layered Uranyl Molybdate [C3H9NH+]4[(UO2)3(MoO4)5] Structurally Based on Mineral-Related Modular Units.
Minerals,
Vol. 10,
Issue. 8,
p.
659.
Kampf, Anthony R.
Olds, Travis A.
Plášil, Jakub
Nash, Barbara P.
and
Marty, Joe
2020.
Pseudomeisserite-(NH4), a new mineral with a novel uranyl-sulfate linkage from the Blue Lizard mine, San Juan County, Utah, USA.
Mineralogical Magazine,
Vol. 84,
Issue. 3,
p.
435.
Chukanov, Nikita V.
and
Vigasina, Marina F.
2020.
Vibrational (Infrared and Raman) Spectra of Minerals and Related Compounds.
p.
741.
Kampf, Anthony R.
Olds, Travis A.
Plášil, Jakub
Marty, Joe
Perry, Samuel N.
Corcoran, Loretta
and
Burns, Peter C.
2021.
Seaborgite, LiNa6K2(UO2)(SO4)5(SO3OH)(H2O), the First Uranyl Mineral Containing Lithium.
American Mineralogist,
Vol. 106,
Issue. 1,
p.
105.
Lahrouch, Florian
Baptiste, Benoit
Dardenne, Kathy
Rothe, Jörg
Elkaim, Erik
Descostes, Michael
and
Gerard, Martine
2022.
Uranium speciation control by uranyl sulfate and phosphate in tailings subject to a Sahelian climate, Cominak, Niger.
Chemosphere,
Vol. 287,
Issue. ,
p.
132139.
Frankland, Victoria L.
Milodowski, Antoni E.
and
Read, David
2022.
Laser-Based Characterisation of the Copper Uranyl Sulphate, Johannite.
Minerals,
Vol. 12,
Issue. 11,
p.
1419.
Plášil, Jakub
Kampf, Anthony R.
Ma, Chi
and
Desor, Joy
2023.
Oldsite, K2Fe2+[(UO2)(SO4)2]2(H2O)8, a new uranyl sulfate mineral from Utah, USA: its description and implications for the formation and occurrences of uranyl sulfate minerals.
Mineralogical Magazine,
Vol. 87,
Issue. 1,
p.
151.
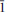 , a = 5.32317(10), b = 11.5105(2), c = 13.5562(10) Å, α = 102.864(7)°, β = 97.414(7)°, γ = 91.461(6)°, V = 801.74(6) Å3, and Z = 2. Crystals are prisms elongated on [100], up to 0.3 mm long, exhibiting the forms {010} and {001}. Meisserite is pale green to yellowish green, translucent to transparent and has a very pale yellow streak. It is brittle, with fair cleavage on {100} and {001}, and uneven fracture. The Mohs hardness is estimated at 2. Meisserite is somewhat hygroscopic and easily soluble in water. The calculated density based on the empirical formula is 3.208 g/cm3. Meisserite exhibits bright yellow green fluorescence under both long- and shortwave UV radiation. The mineral is optically biaxial (–), with α = 1.514(1), β = 1.546(1), γ = 1.557(1) (measured in white light). The measured 2V is 60(2)° and the calculated 2V is 60°. Dispersion is r > v, perceptible, and the optical orientation is X ≈ a, Z ≈ c*. The mineral is pleochroic, with X (colourless) < Y (pale yellow) ≈ Z (pale greenish yellow). The empirical formula of meisserite (based on 19 O a.p.f.u.) is Na5.05(U0.94O2)(SO4)3[SO2.69(OH)1.31](H2O). The Raman spectrum is dominated by the symmetric stretching vibrations of UO22+, SO42– and also weaker O–H stretching vibrations. The eight strongest powder X-ray diffraction lines are [dobs in Å (hkl)Irel]: 13.15 (001) 81, 6.33 (0
, a = 5.32317(10), b = 11.5105(2), c = 13.5562(10) Å, α = 102.864(7)°, β = 97.414(7)°, γ = 91.461(6)°, V = 801.74(6) Å3, and Z = 2. Crystals are prisms elongated on [100], up to 0.3 mm long, exhibiting the forms {010} and {001}. Meisserite is pale green to yellowish green, translucent to transparent and has a very pale yellow streak. It is brittle, with fair cleavage on {100} and {001}, and uneven fracture. The Mohs hardness is estimated at 2. Meisserite is somewhat hygroscopic and easily soluble in water. The calculated density based on the empirical formula is 3.208 g/cm3. Meisserite exhibits bright yellow green fluorescence under both long- and shortwave UV radiation. The mineral is optically biaxial (–), with α = 1.514(1), β = 1.546(1), γ = 1.557(1) (measured in white light). The measured 2V is 60(2)° and the calculated 2V is 60°. Dispersion is r > v, perceptible, and the optical orientation is X ≈ a, Z ≈ c*. The mineral is pleochroic, with X (colourless) < Y (pale yellow) ≈ Z (pale greenish yellow). The empirical formula of meisserite (based on 19 O a.p.f.u.) is Na5.05(U0.94O2)(SO4)3[SO2.69(OH)1.31](H2O). The Raman spectrum is dominated by the symmetric stretching vibrations of UO22+, SO42– and also weaker O–H stretching vibrations. The eight strongest powder X-ray diffraction lines are [dobs in Å (hkl)Irel]: 13.15 (001) 81, 6.33 (0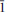 2) 62, 5.64 (0
2) 62, 5.64 (0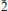 1,020) 52, 5.24 (100,012,
1,020) 52, 5.24 (100,012,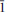 01) 100, 4.67 (101) 68, 3.849 (
01) 100, 4.67 (101) 68, 3.849 (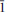
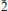 1,102,022) 48, 3.614 (0
1,102,022) 48, 3.614 (0 2,
2,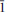
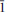 3) 41, and 3.293 (
3) 41, and 3.293 (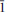 13,004) 43. The crystal structure of meisserite (R1 = 0.018 for 3306 reflections with Iobs > 3σI) is topologically unique among known structures of uranyl minerals and inorganic compounds. It contains uranyl pentagonal bipyramids linked by SO4 groups to form chains. Na+ cations bond to O atoms in the chains and to an SO3OH group and an H2>O group between the chains, thereby forming a heteropolyhedral framework.
13,004) 43. The crystal structure of meisserite (R1 = 0.018 for 3306 reflections with Iobs > 3σI) is topologically unique among known structures of uranyl minerals and inorganic compounds. It contains uranyl pentagonal bipyramids linked by SO4 groups to form chains. Na+ cations bond to O atoms in the chains and to an SO3OH group and an H2>O group between the chains, thereby forming a heteropolyhedral framework.