Published online by Cambridge University Press: 21 February 2018
Marshallsussmanite (IMA2013-067) is a new pyroxenoid mineral from the Wessels mine, Kalahari Manganese Field, Northern Cape Province, South Africa. Marshallsussmanite has ideal formula NaCaMnSi3O8(OH) and triclinic P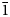 $\bar{1}$ symmetry. Marshallsussmanite forms vitreous pink bladed crystals to 2.1 cm. The mineral shows perfect cleavage on both {100} and {001}. The chemical composition from electron microprobe (average of 20 analyses) and inductively coupled plasma mass spectrometer analysis (average of three analyses) is Li2O 0.43, Na2O 8.06, MgO 0.08, CaO 15.33, MnO 21.79, SiO2 51.71; totalling 97.40 wt.%. The empirical formula, normalized to 3 Si and assuming 1 H apfu is Li0.100Na0.906Ca0.953Mg0.007Mn1.071Si3O8(OH). Unit-cell parameters from single crystal X-ray diffraction are a = 7.7854(4), b = 6.9374(4), c = 6.8516(3) Å, α = 90.683(3)°, β = 94.330(3)°, γ = 102.856(3)°, V = 359.59(3) Å3; Z = 2. The crystal structure refinement converged with Robs = 0.0248 and site occupancy refinement gives crystal chemistry [Na0.948Li0.052][Ca0.793Mn0.207] [Mn0.937Ca0.063]Si3O8(OH). Marshallsussmanite is a single chain silicate with a repeat interval of three tetrahedra (i.e. dreier chain). Marshallsussmanite is a member of the pectolite group of pyroxenoids, which also includes barrydawsonite-(Y), cascandite, pectolite, serandite and tanohataite. Parallel silicate chains form layers, intercalated with well-ordered cation layers. Calcium and Mn both exhibit octahedral coordination, while Na has four bonded interactions in a coordination sphere (radius 3 Å) of seven separate O atoms. Procrystal electron density and bond valence modelling results are compared. The mineral has an unusually strong hydrogen bond with O4⋅⋅⋅O3 separation of 2.458(2) Å. Unlike pectolite and serandite, O4 in marshallsussmanite acts as an H-bond donor and O3 is an H-bond acceptor. Cation ordering in pyroxenoids has a substantial impact on the H position and corresponding H-bonding schemata.
$\bar{1}$ symmetry. Marshallsussmanite forms vitreous pink bladed crystals to 2.1 cm. The mineral shows perfect cleavage on both {100} and {001}. The chemical composition from electron microprobe (average of 20 analyses) and inductively coupled plasma mass spectrometer analysis (average of three analyses) is Li2O 0.43, Na2O 8.06, MgO 0.08, CaO 15.33, MnO 21.79, SiO2 51.71; totalling 97.40 wt.%. The empirical formula, normalized to 3 Si and assuming 1 H apfu is Li0.100Na0.906Ca0.953Mg0.007Mn1.071Si3O8(OH). Unit-cell parameters from single crystal X-ray diffraction are a = 7.7854(4), b = 6.9374(4), c = 6.8516(3) Å, α = 90.683(3)°, β = 94.330(3)°, γ = 102.856(3)°, V = 359.59(3) Å3; Z = 2. The crystal structure refinement converged with Robs = 0.0248 and site occupancy refinement gives crystal chemistry [Na0.948Li0.052][Ca0.793Mn0.207] [Mn0.937Ca0.063]Si3O8(OH). Marshallsussmanite is a single chain silicate with a repeat interval of three tetrahedra (i.e. dreier chain). Marshallsussmanite is a member of the pectolite group of pyroxenoids, which also includes barrydawsonite-(Y), cascandite, pectolite, serandite and tanohataite. Parallel silicate chains form layers, intercalated with well-ordered cation layers. Calcium and Mn both exhibit octahedral coordination, while Na has four bonded interactions in a coordination sphere (radius 3 Å) of seven separate O atoms. Procrystal electron density and bond valence modelling results are compared. The mineral has an unusually strong hydrogen bond with O4⋅⋅⋅O3 separation of 2.458(2) Å. Unlike pectolite and serandite, O4 in marshallsussmanite acts as an H-bond donor and O3 is an H-bond acceptor. Cation ordering in pyroxenoids has a substantial impact on the H position and corresponding H-bonding schemata.
Associate Editor: Michael Rumsey
This new mineral was originally approved by IMA in CNMNC newsletter 18, published in 2013 (IMA2013-067, Min. Mag., 77, 3256). Subsequent to prepublication on the journal's website, new information came to light and in May 2018 the CNMNC of the IMA passed memorandum IMA 18-B: Discreditation of the mineral name ‘marshallsussmanite’ with a reinstatement of the name schizolite (Newsletter 43, Min. Mag., 82, 785). An alternative paper was published by Grice et al. (2019). The original Mineralogical Magazine paper could not be withdrawn as it has already been cited. It is published here to complete the publication record. Readers' attention is directed to the updated paper (Grice et al., 2019, Min. Mag., 83, 473–478).