Published online by Cambridge University Press: 05 July 2018
Zussmanite has been found at only one locality: the Laytonville Quarry, Mendocino County, California. There are, however, present at this locality, two separate, but apparently inter-related minerals that from the evidence of chemistry, and limited diffraction information, appear to be zussmanite-type species. Their relative structural similarities are demonstrated within two rocks from the quarry in which a manganese concentration gradient has allowed ferrous-iron-rich zussmanites to develop partly contiguous overgrowths of one or other of these two minerals, one of which is a new form that has a provisionally determined ideal formula of
KAlMn3−5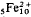 -8Si17O42(OH)14 and that is separated from ideal zussmanite compositions (of the form
-8Si17O42(OH)14 and that is separated from ideal zussmanite compositions (of the form
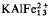 Si17O42(OH)14) by an immiscibility gap. The other ‘zussmanite-type’ mineral has a composition that closely resembles a manganese-rich form of minne-sotaite.
Si17O42(OH)14) by an immiscibility gap. The other ‘zussmanite-type’ mineral has a composition that closely resembles a manganese-rich form of minne-sotaite.
The first zussmanite-type species (ZU2) has been separated and not unambiguous diffraction information obtained of its cell dimensions and powder lines, which are similar to those of zussmanite but appear to have an 8 % smaller cell-base and only a two-layer repeat (as in some of the zussmanite polytypes, and as in the talc structure). It is therefore considered possible that ZU2 has an altered compatibility between the tetrahedral and octa-hedral sheet overlap, perhaps from 13 (as in zussmanite) to 12. Whilst zussmanite appears to be a blueschist-eclogite mineral, ZU2 occurs under conditions at the low-pressure side of the blueschist facies.
An intermediate between zussmanite and the manganoan ‘minnesotaite’ is found in one rock in which the rims of zussmanite have been leached of potassium. As minnesotaite is more of a range of compositions than a structure (there is mounting evidence that it is not a simple talc-analogue) a consideration of the Laytonville manganoan minnesotaite as a zussmanite mineral is not unreasonable.